Table 1.
Apparent IC50 values of the diaryl sulfones and N,N-diarylamine scaffolds for competing with 8-NBD-cAMP in binding EPAC2.
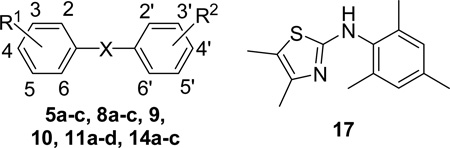 | |||||
|---|---|---|---|---|---|
| Entry | R1 | R2 | X | IC50 (µM) |
Relative Potency (RA)* |
| cAMP | 40 | 1 | |||
| 1 | 4-methyl | 2’,4’,6’-trimethyl | SO2 | 0.5 | 80 |
| 2 | 4-NH2 | 2’-SO3H-4’-NH2 | NH | 18 | 2.2 |
| 5a | 2,4,5-trimethyl | 2’,4’,6’-trimethyl | SO2 | 0.7 | 57.1 |
| 5b | 4-pentyl | 2’,4’,6’-trimethyl | SO2 | >300 | <0.13 |
| 5c | 4-cyclohexyl | 2’,4’,6’-trimethyl | SO2 | >300 | <0.13 |
| 8a | 4-iodo | 2’,4’,6’-trimethyl | SO2 | 4 | 10 |
| 8b | 4-methoxy | 2’,4’,6’-trimethyl | SO2 | 1.9 | 21.1 |
| 9 | 4-(2-fluoro-5-pyridinyl) | 2’,4’,6’-trimethyl | SO2 | >300 | <0.13 |
| 10 | 4-hydroxyl | 2’,4’,6’-trimethyl | SO2 | 13.5 | 3.0 |
| 11a | 4-O-cyclohexyl | 2’,4’,6’-trimethyl | SO2 | >300 | <0.13 |
| 11b | 4-O-piperidinyl-1-Boc | 2’,4’,6’-trimethyl | SO2 | >300 | <0.13 |
| 11c | 4-O-piperidinyl | 2’,4’,6’-trimethyl | SO2 | >300 | <0.13 |
| 11d | 4-O-2-ethylamino | 2’,4’,6’-trimethyl | SO2 | >300 | <0.13 |
| 14a | 4-methyl | 2’,4’,6’-trimethyl | NH | 3.8 | 10.5 |
| 14b | 3,5-dichloro | 2’,4’,6’-trimethyl | NH | 0.9 | 44.4 |
| 14c | 2,5-dichloro | 2’,4’,6’-trimethyl | NH | 0.4 | 100 |
| 17 | NH | >300 | <0.13 | ||
RA = IC50, cAMP/ IC50, compound
