Abstract
Remyelination has to occur to fully regenerate injured spinal cords or brain tissues. A growing body of evidence has suggested that exogenous cell transplantation is one promising strategy to promote remyelination. However, direct injection of neural stem cells or oligodendrocyte progenitor cells (OPCs) to the lesion site may not be an optimal therapeutic strategy due to poor viability and functionality of transplanted cells resulted from the local hostile tissue environment. The overall objective of this study was to engineer an injectable biocompatible hydrogel system as a supportive niche to provide a regeneration permissive microenvironment for transplanted OPCs to survive, functionally differentiate, and remyelinate central nervous system (CNS) lesions. A highly biocompatible hydrogel, based on thiol-functionalized hyaluronic acid and thiol-functionalized gelatin, which can be crosslinked by poly-(ethylene glycol) diacrylate (PEGDA), was used. These hydrogels were optimized first regarding cell adhesive properties and mechanical properties to best support the growth properties of OPCs in culture. Transplanted OPCs with the hydrogels optimized in vitro exhibited enhanced survival and oligodendrogenic differentiation and were able to remyelinate demyelinated axons inside ethidium bromide (EB) demyelination lesion in adult spinal cord. This study provides a new possible therapeutic approach to treat CNS injuries in which cell therapies may be essential.—Li, X., Liu, X., Cui, L., Brunson, C., Zhao, W., Bhat, N. R., Zhang, N., Wen, X. Engineering an in situ crosslinkable hydrogel for enhanced remyelination.
Keywords: biomaterials, oligodendrocyte progenitor cells, transplantation, spinal cord regeneration
Axonal demyelination is an inevitable component in many types of neural tissue injury, including spinal cord injury and traumatic brain injury. Remyelination has to occur to cure these diseases and to fully regenerate injured spinal cords or brain tissues. At present, there are no effective therapies that promote remyelination. Since remyelination involves the generation of new mature oligodendrocytes, current research strategies for remyelination in animal models have been focused on oligodendrogenic stem/progenitor cells of both endogenous and exogenous origins (1, 2). Previous studies on the role of oligodendrocytes and oligodendrocyte progenitors in central nervous system (CNS) remyelination have evidenced the dominant contribution of oligodendrocyte progenitor cells (OPCs) to remyelinate spinal cord lesions. Although spontaneous remyelination mediated by endogenous OPCs can be a highly effective regenerative process, this response is incomplete and fails over time due to the limited availability, migratory capacity, and myelinating ability (3, 4). In contrast, cell transplantation (exogenous therapies) using stem cells or progenitors, including OPCs (5), induced pluripotent stem cells (6), mesenchymal stem cells (7), neural stem cells (8), embryonic stem cell-derived progenitors (9), and olfactory ensheathing cells (10), have all been shown to achieve some remyelination in demyelinated adult CNS. These studies have suggested that remyelination is particularly amenable to cell-based therapies.
However, direct injection of neural stem cells or OPCs to the lesion site may not be an optimal therapeutic strategy due to poor viability and functionality of transplanted cells resulting from the local hostile tissue environment (11, 12). The fate of transplanted cells is strongly influenced by the type of injuries and local microenvironmental signals (biomechanical and biomolecular signals). As to remyelination failure, the scarring and inflammatory tissue environment at the demyelinating site may be deleterious to the survival and directed differentiation of transplanted cells with the presence of differentiation block of oligodendroglial progenitors in the spinal cord injury lesion sites (13). Control over stem cell trafficking, survival, proliferation, and differentiation within a complex demyelinating in vivo milieu continues to be extremely challenging.
An appealing approach to address the issues in transplantation is the development and the use of 3-dimensional (3D) biomaterial systems that create specialized niches supporting the transplanted cells (17–21). Key niche components and interactions include signaling molecules, cell-cell contacts, and cell-matrix adhesions. The interplay of these niche factors is particularly important to comprehend if any desired cell response is to be made robust for therapy. A biomaterial carrier for cell transplantation may serve as a protective corona around the cells to ward off the hostile in vivo lesion environment, promoting cell survival, proliferation, differentiation, and organization (22, 23). Hydrogels are crosslinked hydrophilic polymer networks with physical characteristics similar to soft tissues and high permeability, which facilitates exchange of oxygen, nutrients, and other water-soluble metabolites, as well as cell infiltration, angiogenesis, and tissue growth. For many clinical uses, injectable in situ crosslinkable hydrogels would be strongly preferred, because the injectable material could be formed into any desired shape at the site of injury, the crosslinkable polymer mixture would adhere to the tissue during gelation, and the mechanical interlocking arising from irregular interfaces would strengthen the tissue-hydrogel integration.
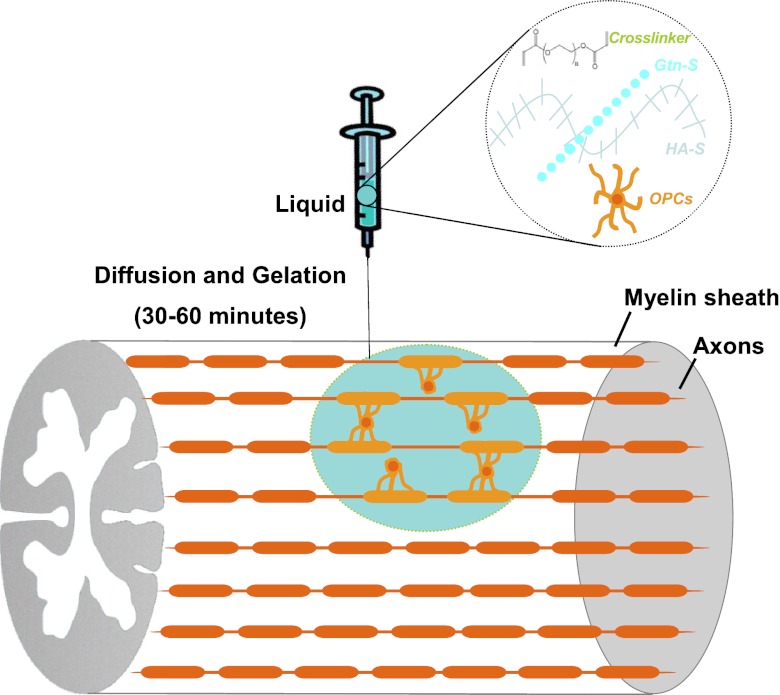
The overall objective of this study was to engineer an injectable biocompatible hydrogel system as a supportive niche to provide a regeneration-permissive microenvironment for transplanted OPCs to survive, functionally differentiate, and remyelinate CNS lesions. A highly biocompatible hydrogel is based on thiol-functionalized hyaluronic acid (HA-S) and human recombinant thiol-functionalized gelatin (Gtn-S), which can be crosslinked by poly-(ethylene glycol) diacrylate (PEGDA) through Michael-type addition reaction. This hydrogel system was engineered regarding cell adhesive properties and mechanical properties to best support the growth properties of OPCs in culture. Transplanted OPCs with the hydrogels optimized in vitro exhibited enhanced survival and oligodendrogenic differentiation, and were able to remyelinate demyelinated axons inside ethidium bromide (EB) demyelination lesions in adult spinal cord. The schematic drawing of OPCs transplanted with in situ crosslinkable hydrogels for spinal cord remyelination is shown in Fig. 1. This study is the first demonstrated extensive remyelination with transplanted progenitor cells and provides a new possible treatment for demyelination-related diseases and CNS injuries in which cell therapies may be essential.
Figure 1.
Schematic drawing of OPCs transplanted with in situ crosslinkable hydrogels for spinal cord remyelination.
MATERIALS AND METHODS
In situ crosslinkable hydrogels
HA-S solution (Glycosan BioSystems, Salt Lake City, UT, USA) and human recombinant Gtn-S solution were prepared in sterile deionized distilled water under aseptic conditions. A 10% (w/v) PEGDA stock solution was prepared by dissolving PEGDA powder (Laysan Bio, Arab, AL, USA) in phosphate-buffered saline (PBS).
Rheological characterization of hydrogels
To test the mechanical properties of the formed hydrogel series, HA-S solution (1% w/v) and Gtn-S solution (1%, w/v; Gtn ratio: 75, 50, 25, and 0%) with varying PEGDA concentrations (10, 5, 2.5, 1, and 0.5%) were inspected with an oscillatory shear rheometer (AR 1000; TA Instruments, New Castle, DE, USA). The time sweep was performed to monitor the in situ gelation at 37°C, recording the temporal evolution of shear storage modulus, G′, and the shear loss modulus, G″. Frequency sweep tests were used to obtain information about the stability of hydrogel structures (24). The stress sweep was set up by holding the frequency 1 Hz constant while increasing the stress level from 1 to 10 Pa. The applied range of 1–10 Pa was found to be safe-for-use from a prior experiment where we determined the linear viscoelastic region (LVR) profiles of the hydrogels by shearing them until structural breakdown. Oscillatory stress sweep allowed determination of G′ of hydrogels.
The elastic modulus, E′, can be evaluated by E′ = 2G′ (1+γ). When a material can be assumed to be incompressible, its Poisson's ratio, γ, approaches 0.5, and this relationship approaches E′ = 3G′. This assumption for hydrogels is supported by a research showing that n for polyacrylamide hydrogels is nearly 0.5 and because these hydrogels are typically used under very low strain conditions (25).
In addition to using rheometer to test the mechanical properties of hydrogels, fresh adult rat CNS tissues were examined by rheometer using the same rheological protocol.
OPC culture
The CG4 cell, originally described by Louis et al. (26), displays the essential characteristics of OPCs, including response to growth factors and differentiation into oligodendrocytes in the absence of mitogenic factors, and thus represents a useful model to study oligodendrocyte development and differentiation. The cells were maintained with serum-free growth medium containing transferrin (50 μg/ml), insulin (50 ng/ml), sodium selenite (20 nM), and triiodo-l-thyronine (30 nM) supplemented with platelet-derived growth factor (PDGF; 10 ng/ml), and fibroblast growth factor 2 (FGF2; 10 ng/ml).
For 2-dimensional (2D) culture (on the surface of hydrogels), HA-S solution and Gtn-S solution (Gtn ratio: 75, 50, 25, and 0%) with different PEGDA concentrations (10, 5, 2.5, 1, and 0.5%) were added to 48-well culture plate and cured overnight to stabilize PEGDA-mediated crosslinking. OPCs were seeded on the surface of hydrogels at 5 × 103 cells/cm2. As to 3D culture (inside hydrogels), to avoid cells precipitating onto the surface of plate during the hydrogel formation, we coated the plate with hydrogels overnight and then mixed OPCs with hydrogel precursor solution (1×104 cells/well) and seeded on top of preformed hydrogels.
Cell viability
Viability of cells was examined using a live/dead viability kit (L-7013; Molecular Probes, Eugene, OR, USA), which is a 2-color fluorescent assay based on differential permeability of live and dead cells and allows preservation of the distinctive staining pattern for a couple of hours after postfixation with 4% (w/v) glutaraldehyde. Live cells were stained with green fluorescent SYTO 10, and dead cells with compromised cell membranes were stained with red fluorescent ethidium homodimer-2. A confocal laser microscope (TCS SP5; Leica Microsystems, Bannockburn, IL, USA) was used to capture the images of the live/dead cell staining patterns.
Cell morphology
Morphology of cells was examined by immunocytochemistry. The OPCs were fixed with 4% (w/v) paraformaldehyde for 30 min, treated with 5% goat serum in PBS to block nonspecific reactivity, and incubated overnight at 4°C with first antibody, such as A2B5 (MsIgM, ab5321; Abcam, Cambridge, MA, USA). After being washed with PBS 3 times, the samples were incubated with the second antibody for 3 h at room temperature. Then, the nuclei were counterstained with 4′,6-diamidino-2-phenylindole, dihydrochloride (DAPI) for 30 min. The samples were imaged using a Leica TCS SP5 laser scanning confocal microscope. For each well, 5 images were taken from different regions.
Cell adhesion and proliferation
Proliferation of cells was examined using the Click iT-EdU cell proliferation assay (C10085; Invitrogen, Grand Island, NY, USA). 5-Ethynyl-2′-deoxyuridine (EdU) is a nucleoside analog of thymidine and is incorporated into DNA during active DNA synthesis. Detection is based on a click reaction, a copper (Cu+)-catalyzed covalent reaction between an azide and an alkyne. EdU was applied directly to the cells on the surface of hydrogels for 4 h before fixation with 3.7% (w/v) formaldehyde in PBS. Fluorescin-labeled anti-EdU monoclonal antibody was applied to identify proliferating cells. The total attached cells were detected by propidium iodide (PI) staining. The samples were imaged using a Leica TCS SP5 laser scanning confocal microscope. For each well, 5 images were taken from different regions. The percentage of EdU cells in the population was calculated and compared among groups.
Demyelinating lesion and transplantation of OPCs
We have chosen an experimental model of toxin-induced focal demyelination using EB to demyelinate specific CNS tracts in a dose-dependent manner (27). A total of 28 female nude rats (150–200 g; Charles River Laboratories, Charleston, SC, USA) were used throughout the study. An EB model of focal demyelination induced by injecting EB at the left ventral white matter of the thoracic spinal cord was conducted. Briefly, rats were anesthetized with 4% (w/v) chloral hydrate (10 ml/kg). Dorsal laminectomies were performed at the T9/T10 vertebral level, and the dura was opened transversely at two sites 2 mm apart. Unilateral injections (1 μl/ injection, n=3) of 0.1 mg/ml EB were delivered at stereotactic coordinates for the ventrolateral funiculus (VLF) of the thoracic spinal cord (0.7 mm lateral to midline and depth of 1.5 mm). With the aid of an operating microscope, the EB was stereotactically injected at a rate of 0.5 μl/min using a Hamilton syringe with a 32-gauge customized needle and a motorized microinjector (model 780310; Stoelting, Wood Dale, IL, USA).
All transplantations were performed 7 d postsurgery. Following induction of anesthesia, T9/T10 laminectomy site was reexposed. There were 3 treatment groups: OPCs only (1×105 cells/μl); optimized hydrogel only; and OPCs with optimized hydrogels. OPCs were delivered at a rate of 0.5 μl/min directly into the EB lesion using the same stereotactic coordinates as described above. The needle was left in place for an additional 2 min to prevent backflow of cells. PBS was delivered to the control group. Surgical interventions and perioperative care were provided in strict accordance with the Public Health Service Policy on Humane Care and Use of Laboratory Animals and were approved by the Medical University of South Carolina Institutional Animal Care and Use Committee.
Tissue processing and immunohistochemistry
To evaluate OPCs transplanted following demyelination, animals were sacrificed at 4 wk post-transplantation. Animals were deeply anesthetized with 4% chloral hydrate (10 ml/kg) and transcardially perfused with 300 ml PBS followed by 300 ml of 4% paraformaldehyde in PBS. A 0.8-cm segment of spinal cord encompassing the transplant site was dissected and postfixed overnight at 4°C and then transferred to 30% sucrose for cryoprotection (48 h). Specimens were cut transversely at the epicenter of the lesion and mounted with the cut surfaces facing down in tissue-freezing medium. After tissue embedding, 20-μm-thick transverse sections were cut on a Leica CM3050 cryostat and mounted on microscope slides.
For immunostaining, sections were permeabilized with 0.5% Triton X-100 and blocked with 4% normal goat serum in PBS for 2 h. Primary antibodies were then applied overnight at 4°C. The following primary antibodies were used: glial fibrillary acidic protein (GFAP) for astrocytes (1:1000; Dako, Carpinteria, CA, USA), myelin basic protein (MBP) for mature oligodendrocytes (1:500, Abcam, Cambridge, MA, USA), and β III tubulin for axons (1:1000; Sigma, St. Louis, MO, USA). Cy2 and cy3 affinity secondary antibodies and goat anti-mouse and rabbit were used at 1:400 (Jackson ImmunoResearch Laboratories, West Grove, PA, USA). The specimens were imaged using a Leica TCS SP5 laser scanning confocal microscope.
Statistical analysis
Data are presented as the mean ± sd for each group. One-way ANOVA was performed to determine the effect of hydrogel property and hydrogel use on the outcome using SPSS software (SPSS Inc., Chicago, IL, USA). Statistical significance was accepted at values of P < 0.05.
RESULTS AND DISCUSSION
Mechanical properties of hydrogels
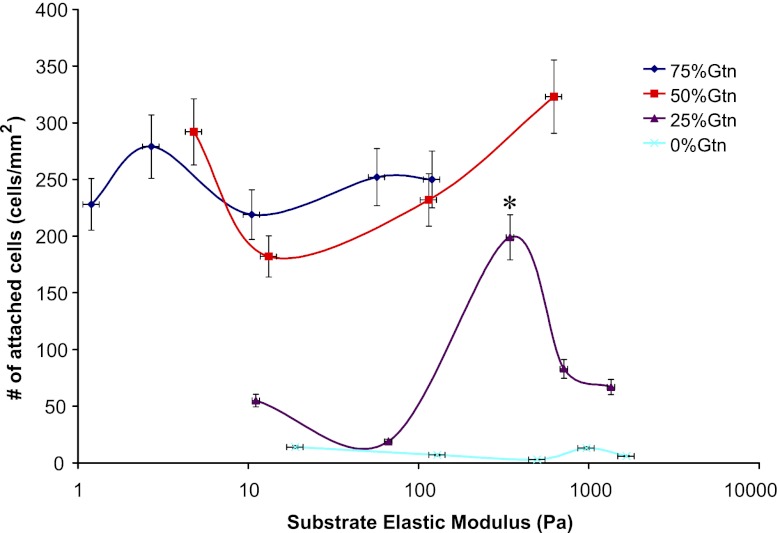
To develop CNS-compatible hydrogels to be used as cell carriers in the adult CNS, we need to ensure the mechanical compliance of the hydrogels with native CNS tissue. The storage modulus G′ of rat CNS is ∼40 Pa or elastic modulus E′ of ∼120 Pa, as characterized by rheometer (Supplemental Fig. S1). Note that the storage modulus G′ always exceeds the loss modulus G″, indicating that the adult rat CNS has an elasticity-dominant rather than viscosity-dominant mechanical property, which is in agreement with previous studies (28). The mechanical properties of this hydrogel system can be controlled by varying a few parameters, such as the concentrations of HA-S and Gtn-S, the ratio of HA-S to Gtn-S, PEGDA concentration, and so on. In this study, the effects of two parameters, i.e., Gtn-S percentage, and PEGDA concentration on hydrogel properties and OPC behaviors are examined. Each of the two parameters was examined independently while keeping other variables constant. By varying the gelatin percentages, or PEGDA concentrations, hydrogels with E′ ranging from 1 to 1600 Pa, which spans the range of that of native CNS tissue (120 Pa), can be achieved (Fig. 2). The gelatin percentage and PEGDA concentration exhibited opposing effects on the hydrogel elastic modulus, i.e., E′ increases as a function of decreasing Gtn-S percentage, whereas E′ increases with increasing PEGDA concentration. Since PEGDA acts as the crosslinker for the hydrogel system, E′ increases with the increasing PEGDA concentration. The cell adhesive component in the hydrogel Gtn-S contributes to the viscosity rather than the elasticity of the hydrogels.
Figure 2.
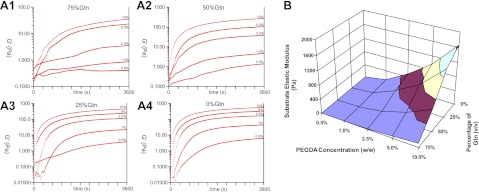
Time sweep of G′ as a function of PEGDA concentration (10, 5, 2.5, 1, and 0.5%) and Gtn-S percentage (75, 50, 25, and 0%). A1) 75%. A2) 50%. A3) 25%. A4) 0%. B) Elastic modulus of the hydrogels as a function of PEGDA concentration and Gtn-S percentage.
Optimizing hydrogels for OPC culture
Extracellular matrix (ECM) is an important component for the stem cell niche and regulating stem cell behavior and functions. Biomaterials can be used to create a niche to support stem cell survival in vivo by providing the biochemical and biomechanical environments for the tissues to be regenerated. HA has long been recognized as an important ECM component for CNS tissues. Inclusion of HA imparts hydrophilic network structures to the hydrogels. However, HA is extremely hydrophilic and polyanionic, which prevents cell attachment and limits its ability to support cell growth and tissue remodeling. To promote cell growth and function, a cell adhesive component, human recombinant gelatin, was incorporated in the hydrogel. The effects of biochemical and biomechanical properties of the hydrogels on OPC attachment, proliferation, and directed differentiation were examined in vitro and in vivo.
The effect of the adhesive component Gtn-S in the hydrogels on OPC behavior, such as attachment, morphology, and proliferation, was evaluated by culturing OPCs on the surfaces of the hydrogels with different gelatin content and elastic modulus. When compared with that of the elastic modulus, Gtn-S content in the hydrogels exerted greater effect on cell attachment, as evidenced by magnitude-higher numbers of attached OPCs on hydrogels with high Gtn-S content (>50%) relative to those with low Gtn-S content (Fig. 3). At low Gtn-S percentages (25%), the numbers of attached OPCs were dependent on hydrogel elastic modulus, manifested by the appearance of a peak on the curve (P<0.05). In comparison, at high Gtn-S percentages (50%), the OPC attachment was independent of the elastic modulus, with the curves almost flattened over the elastic modulus. These results suggest that when adhesive components are present at sufficiently high percentages in the hydrogels, the cell adhesive component may dominate over material mechanical properties and dictate cell attachment. There was no significant difference in OPC attachment between the 50 and 75% gelatin groups, perhaps due to the saturation in the presentation of surface binding domains by high-percentage Gtn-S in the hydrogels. Given the range of elastic modulus achieved with each value of gelatin percentage, OPC attachment was best favored on the surfaces of the hydrogels with 50% Gtn-S.
Figure 3.
Number of attached OPCs on the surfaces of the hydrogels as a function of hydrogel elastic modulus and Gtn-S content. On the hydrogels with 25% Gtn-S, OPCs preferred to attach on hydrogels of medium stiffness. *P < 0.05.
Parallel investigations of the morphologies of attached OPCs on hydrogels of the same elastic modulus (10 Pa) but with different gelatin percentages revealed no significant difference among the groups (Supplemental Fig. S2). All the attached cells displayed spreading cytoskeletons (red is the staining for A2B5, a specific surface marker for OPCs). The proliferation of attached OPCs on hydrogel surfaces was examined using Click iT-EdU kit (Supplemental Fig. S3). All the attached OPCs on the hydrogel surfaces were stained in red using PI, while the proliferating OPCs were stained in blue using a Click iT-EdU 647. Regardless of the gelatin percentage, attached OPCs exhibited an approximate 5% proliferation rate (blue-to-red cell ratio).
Hydrogel stiffness affects OPC behaviors
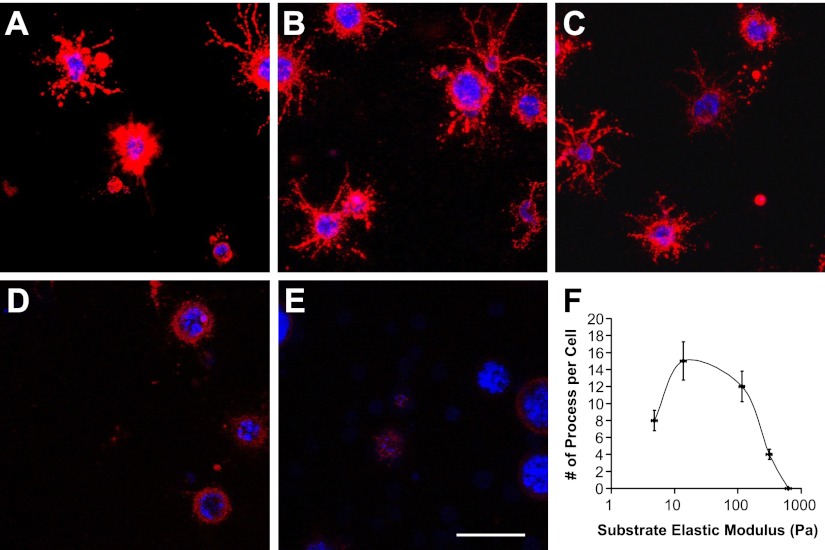
OPCs were cultured on the surfaces and inside the hydrogels, representing 2D and 3D culture conditions, respectively. At 3 d in 2D culture, OPCs exhibited a biphasic change in morphology over increasing elastic modulus of the hydrogels (Fig. 4A1–E1). On soft hydrogels (Fig. 4A1, B1; 4.8 and 13.8 Pa), OPCs displayed round morphology with very few spreading processes. On hydrogels of medium stiffness (Fig. 4C1, 116 Pa), OPCs were primarily spreading, resembling the natural morphology of OPCs in the body, which is important for oligodendrocytes to spirally enwrap axons, forming multilamellar myelin sheaths. On stiff hydrogels (Fig. 4D1, E1; 312 and 624 Pa), cell spreading was diminished over hydrogel stiffness, with OPCs assuming round morphology in cell aggregates. A similar trend of biphasic change was observed in cell morphology vs. hydrogel stiffness at 7 d in 2D culture (Fig. 4A2–E2). OPCs were increasingly spreading with sprouting processes within low to medium range of hydrogel elastic modulus and were then progressively aggregated and assumed round morphology within medium to high range of hydrogel elastic modulus. The morphologies of OPCs were quantified by averaging the number of process from single OPC. OPCs on the hydrogel of medium stiffness (116 Pa, which is close to that of native CNS tissue) expressed highest number of sprouting processes at both 3 and 7 d (Fig. 4F; P<0.05).
Figure 4.
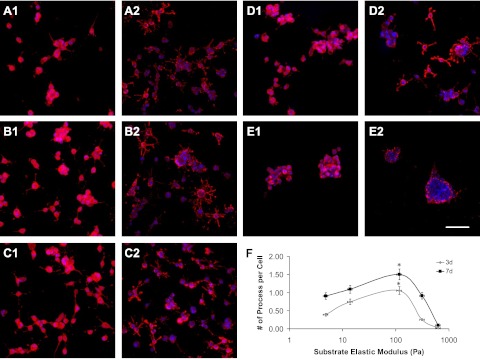
Morphology of cultured OPCs on the surfaces of the hydrogels (50% Gtn-S) with different elastic modulus at 3 d (A1–E1) and 7 d (A2–E2). A1, A2) 4.8 Pa. B1, B2) 13.8 Pa. C1, C2) 116 Pa. D1, D2) 312 Pa. E1, E2) 624 Pa. F) Plot of the sprouting processes from OPCs as a function of hydrogel elastic modulus. OPCs possess largest sprouting processes on the hydrogels of medium stiffness at both 3 and 7 d. Red, A2B5 staining for OPCs; blue, DAPI staining for cell nuclei. Scale bar = 150 μm. *P < 0.05.
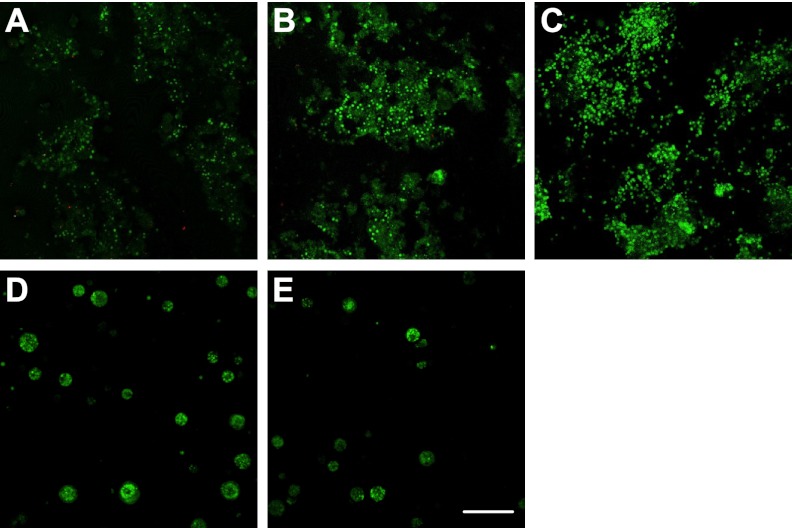
In parallel, OPCs survived in 3D culture (i.e., inside the hydrogels). The viability of OPCs was high (>98%) regardless of the hydrogel elastic modulus (Fig. 5). However, OPCs expressed normal oligomorphology with a hydrogel elastic modulus < 120 Pa (Fig. 6A–C). Hydrogels with higher elastic modulus led to spherical structures of OPCs without sprouting processes (Fig. 6D, E). These hydrogels may be too stiff to allow processes sprouting and extension for the OPCs (29). As a function of the hydrogel elastic modulus, the number of processes of OPCs cultured inside hydrogels decreased when the modulus is >120 Pa (Fig. 6F).
Figure 5.
Live/dead (green/red) staining of cultured OPCs inside the hydrogels (50% Gtn-S) with different elastic modulus at 7 d. A) 4.8 Pa. B) 13.8 Pa. C) 116 Pa. D) 312 Pa. E) 624 Pa. Scale bar = 150 μm.
Figure 6.
Morphology of cultured OPCs inside hydrogels (50% Gtn-S) with different elastic modulus at 7 d. A) 4.8 Pa. B) 13.8 Pa. C) 116 Pa. D) 312 Pa. E) 624 Pa. F) Plot of OPC sprouting processes as a function of hydrogel elastic modulus. OPCs expressed large sprouting processes inside hydrogels with lower stiffness. Red, A2B5 staining for OPCs; blue, DAPI staining for cell nuclei. Scale bar = 50 μm.
Cell adhesion and proliferation are important indexes to evaluate the appropriateness of a cell carrier to support cell functions. As a function of the hydrogel elastic modulus, the proliferation rate of cultured OPCs on the surfaces of the hydrogels increased when the modulus is <120 Pa and decreased when the modulus is >120 Pa (Fig. 7). Hydrogel with an elastic modulus of 120 Pa, which is close to that of native CNS tissue, best supports OPC proliferation (Fig. 7F; P<0.05).
Figure 7.
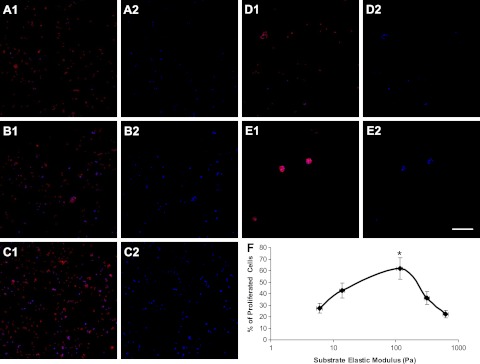
Proliferation of cultured OPCs on the surfaces of hydrogels (50% Gtn-S) with different elastic modulus. A1, A2) 4.8 Pa. B1, B2) 13.8 Pa. C1, C2) 116 Pa. D1, D2) 312 Pa. E1, E2) 624 Pa. F) Plot of the rate of OPC proliferation as a function of hydrogel elastic modulus. On the surfaces of hydrogels (50% Gtn-S) of different stiffness, OPCs exhibited the highest proliferation rate on the hydrogels of medium stiffness Red, PI staining for attached cells (A1–E1); blue, Click iT-EdU 647 staining for proliferating cells (A2–E2). Scale bar = 300 μm. *P < 0.05.
OPC transplantation with optimized hydrogel as carrier
OPCs are most often referred to a population of adult CNS stem/precursor cells that are capable of differentiating into mature oligodendrocytes (30). Lines of evidence on the contribution of OPCs as the major source of remyelinating oligodendrocytes have come from studies through in vivo tracing of both endogenous OPCs (3, 31) and transplanted OPCs. Complementary studies on mature oligodendrocytes have suggested their inability to contribute to remyelination in adult CNS, further supporting the role of OPCs as the primary cell source for functional remyelination. To remyelinate the axons, OPCs have to establish contact with the axons to be remyelinated, express myelin genes, and form myelin membranes, which then ensheath neuronal axons. In addition to a deficiency of differentiation-inducing factors, a demyelinating tissue environment presents inhibitory factors that are responsible for differentiation failure (13, 32). The capacity of OPCs to proliferate and migrate when transplanted into demyelinated areas is also strongly dependent on the stage of the cells, with early stage OPCs remyelinating better across a wide area than do late-stage ones. In the present study, we used CG4 cells, an early stage adult OPC cell line.
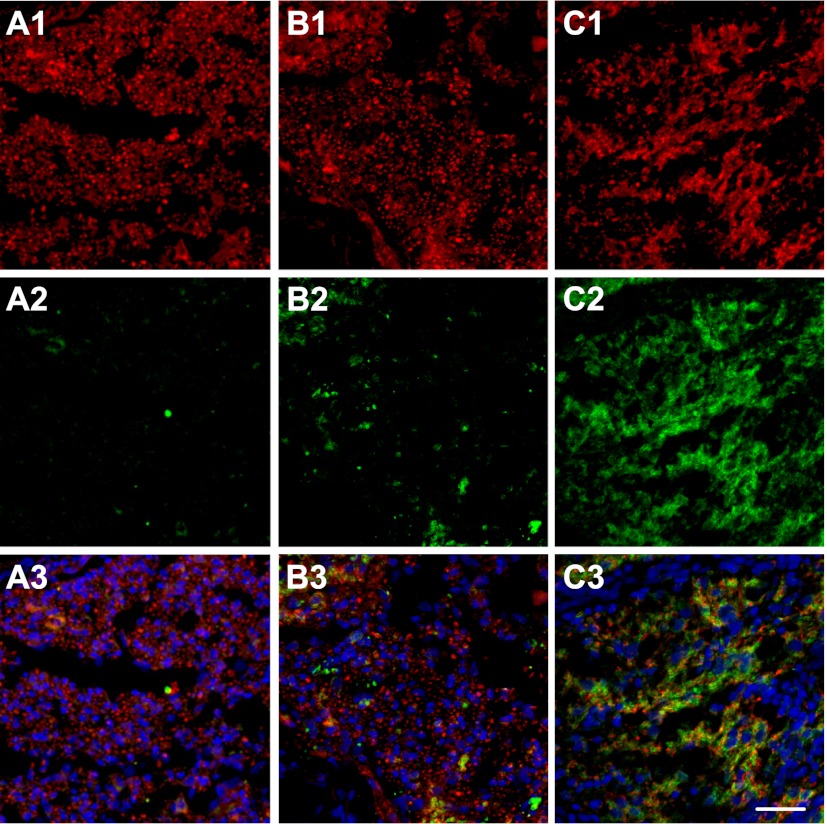
The demyelination model was established by the injection of EB at the left ventrolateral funiculus of the rat spinal cords, which leaves a population of demyelinated axons in a glial-depleted environment. OPCs were transplanted to the EB-demyelinated lesion area in two treatment groups, i.e., OPCs only and OPCs with optimized hydrogel carrier. Examination of the lesion site following OPC transplantation revealed remarkable difference in OPC survival, differentiation, and remyelination between the two cell treatment groups. When compared with the untreated control group (demyelinated lesion with PBS injection) in which the demyelinated area remained as a substantial cavity (data not shown), both cell treatment groups displayed cell populations at the lesion site, as evidenced by the presence of cell nuclei (Fig. 8B, C). In particular, the lesion site in the OPCs with optimized hydrogel group was much more densely repopulated by MBP-positive oligodendrocytes (Fig. 8C2, indicated by the presence of numerous red circle-shaped MBP-positive structure typical of normal myelination) when compared with that in the OPCs only treatment group, where fewer red-stained MBP-positive cells were seen within the lesion site (Fig. 8B2, D; P<0.05). A few GFAP-positive astrocytes were seen in both cell treatment groups. The absence of glial cells at the lesion site in the hydrogel only group suggests that the cells seen at the lesion in the two cell treatment groups were primarily transplanted cells rather than of endogenous origin (Fig. 8A2). The hydrogel carrier has protected transplanted OPCs within a hostile demyelinated lesion environment for better survival and overcomes environmental cues that normally restrict the differentiation potential of transplanted OPCs, may have facilitated OPCs differentiation into mature oligodendrocytes. These mature oligodendrocytes have remyelinated demyelinated axons in the lesion site, which have been demonstrated in Fig. 9.
Figure 8.
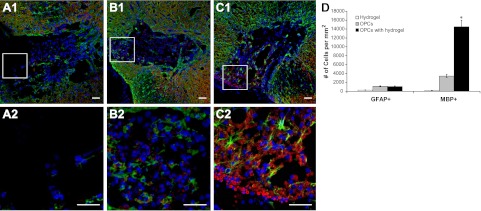
Examination of transplanted OPCs in the lesion sites with 3 treatment groups. A1, A2) Optimized hydrogels only. B1, B2) OPCs only. C1, C2) OPCs with optimized hydrogels. Panels A2, B2, C2 show enlarged views of boxed areas in panels A2, B2, C2, respectively. D) Quantity analysis of transplanted OPCs. Green, GFAP staining for astrocytes; red, MBP staining for myelin; blue, DAPI staining for cell nuclei. Scale bars = 100 μm. *P < 0.05.
Figure 9.
Examination of remyelination in the lesion sites with 3 treatment groups. A1–A3) Optimized hydrogels only. B1–B3) OPCs only. C1–C3) OPCs with optimized hydrogels. Red. β III tubulin staining for axons (A1–C1); green, MBP staining for myelin (A2–C2); blue, DAPI staining for cell nuclei (A3–C3). Scale bar = 200 μm.
The use of hydrogels, which can create a specialized niche for transplanted cells, is a very promising tool for treating neurological disorders and injuries. Hydrogels transplanted with neural stem cells seem to promote neuronal differentiation, enhance the elaboration of neural processes, foster the reformation of tissue, promote connectivity, and reduce inflammation and scarring (33). Similar to our results, Zhong et al. (34) used hyaluronic acid-based hydrogels to transplant neural stem/progenitor cells for recovery after stroke. When compared with stem cell injection without hydrogel support, the hydrogel enhanced survival of stem cells in the normally hostile environment and also prevented inflammatory infiltration (34). Uemura et al. (35) has shown that Matrigel was able to effectively support embryonic stem cell-derived neural precursor cell survival, migration, and neurite outgrowth in vitro and also to promote proliferation of grafted cells in vivo, which resulted in larger graft volume and an increase in the number of TH-positive dopaminergic neurons within the graft (35). Tysseling-Mattiace et al. (36) has developed self-assembly of peptide amphiphile molecules to form cylindrical nanofiber hydrogels. These hydrogels have been shown to inhibit glial scar formation, enhance differentiation of neural progenitor cells, and promote axon elongation, which ultimately resulted in behavioral improvements after spinal cord injury in mice once implanted in vivo (36).
CONCLUSIONS
A hydrogel system has been developed as a candidate material for OPC niche formation. This study is the first demonstrated extensive remyelination with transplanted progenitor cells in vivo. The hydrogel harnesses the potential of exogenous OPCs for CNS remyelination by promoting the survival, natural morphology, proliferation, oligodendrogenic differentiation, and myelin formation of transplanted OPCs in a demyelinated CNS lesion environment. This work exemplifies the efforts to develop material equivalents to stem/precursor cell niche through engineering strategies based on an integration of material properties with neural compatible biochemical and biomechanical properties. This study represents one of the first to engineer neural compatible hydrogels as OPC niche to promote remyelination in vivo. The results indicate that human recombinant gelatin benefits OPC attachment without significant effect on OPC proliferation and morphology. Hydrogel elastic modulus affects the overall cell morphology and an optimal range of elastic modulus exists that best supports the natural oligo-morphology and proliferation of OPCs in both 2D and 3D cultures. OPCs transplanted with hydrogels optimized with cell adhesive properties and mechanical properties as determined in the in vitro experiments exhibited enhanced survival and oligodendrogenic differentiation and were able to remyelinate demyelinated axons inside demyelination lesion in adult spinal cord. This study provides a new therapeutic approach to treat trauma/disease conditions in which cell therapies may be essential.
Supplementary Material
Acknowledgments
This work was supported by U.S. National Institutes of Health grant R01-NS050243, National Science Foundation grant 0748129, and American Heart Association grant 10PRE4280017.
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- 2D
- 2-dimensional
- 3D
- 3-dimensional
- CNS
- central nervous system
- DAPI
- 4′,6-diamidino-2-phenylindole, dihydrochloride
- E′
- elastic modulus
- EB
- ethidium bromide
- EdU
- 5-ethynyl-2′-deoxyuridine
- FGF2
- fibroblast growth factor 2
- G′
- shear storage modulus
- G″
- shear loss modulus
- GFAP
- glial fibrillary acidic protein
- Gtn-S
- thiol-functionalized gelatin
- HA-S
- thiol-functionalized hyaluronic acid
- LVR
- linear viscoelastic region
- MBP
- myelin basic protein
- OPC
- oligodendrocyte progenitor cell
- PBS
- phosphate-buffered saline
- PDGF
- platelet-derived growth factor
- PEGDA
- poly-(ethylene glycol) diacrylate
- PI
- propidium iodide
REFERENCES
- 1. Yang J., Rostami A., Zhang G. X. (2009) Cellular remyelinating therapy in multiple sclerosis. J. Neurol. Sci. 276, 1–5 [DOI] [PubMed] [Google Scholar]
- 2. Jadasz J., Aigner L., Rivera F., Küry P. (2012) The remyelination philosopher's stone: stem and progenitor cell therapies for multiple sclerosis. Cell Tissue Res. 349, 331–347 [DOI] [PubMed] [Google Scholar]
- 3. Deverman B. E., Patterson P. H. (2012) Exogenous leukemia inhibitory factor stimulates oligodendrocyte progenitor cell proliferation and enhances hippocampal remyelination. J. Neurosci. 32, 2100–2109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kotter M. R., Stadelmann C., Hartung H.-P. (2011) Enhancing remyelination in disease—can we wrap it up? Brain 134, 1882–1900 [DOI] [PubMed] [Google Scholar]
- 5. Cao Q., He Q., Wang Y., Cheng X., Howard R. M., Zhang Y., DeVries W. H., Shields C. B., Magnuson D. S. K., Xu X.-M., Kim D. H., Whittemore S. R. (2010) Transplantation of ciliary neurotrophic factor-expressing adult oligodendrocyte precursor cells promotes remyelination and functional recovery after spinalcord injury. J. Neurosci. 30, 2989–3001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tsuji O., Miura K., Okada Y., Fujiyoshi K., Mukaino M., Nagoshi N., Kitamura K., Kumagai G., Nishino M., Tomisato S., Higashi H., Nagai T., Katoh H., Kohda K., Matsuzaki Y., Yuzaki M., Ikeda E., Toyama Y., Nakamura M., Yamanaka S., Okano H. (2010) Therapeutic potential of appropriately evaluated safe-induced pluripotent stem cells for spinal cord injury. Proc. Natl. Acad. Sci. 107, 12704–12709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gordon D., Pavlovska G., Uney J. B., Wraith D. C., Scolding N. J. (2010) Human mesenchymal stem cells infiltrate the spinal cord, reduce demyelination, and localize to white matter lesions in experimental autoimmune encephalomyelitis. J. Neuropathol. Exp. Neurol. 69, 1087–1095 [DOI] [PubMed] [Google Scholar]
- 8. Einstein O., Friedman-Levi Y., Grigoriadis N., Ben-Hur T. (2009) Transplanted neural precursors enhance host brain-derived myelin regeneration. J. Neurosci. 29, 15694–15702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Aharonowiz M., Einstein O., Fainstein N., Lassmann H., Reubinoff B., Ben-Hur T. (2008) Neuroprotective effect of transplanted human embryonic stem cell-derived neural precursors in an animal model of multiple sclerosis. PLoS One 3, e3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sasaki M., Lankford K. L., Radtke C., Honmou O., Kocsis J. D. (2011) Remyelination after olfactory ensheathing cell transplantation into diverse demyelinating environments. Exp. Neurol. 229, 88–98 [DOI] [PubMed] [Google Scholar]
- 11. Kuhlmann T., Miron V., Cuo Q., Wegner C., Antel J., Bruck W. (2008) Differentiation block of oligodendroglial progenitor cells as a cause for remyelination failure in chronic multiple sclerosis. Brain 131, 1749–1758 [DOI] [PubMed] [Google Scholar]
- 12. Hur E.-M., Yang I. H., Kim D.-H., Byun J., Saijilafu, Xu W.-L., Nicovich P. R., Cheong R., Levchenko A., Thakor N., Zhou F.-Q. (2011) Engineering neuronal growth cones to promote axon regeneration over inhibitory molecules. Proc. Natl. Acad. Sci. 108, 5057–5062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang Y., Cheng X., He Q., Zheng Y., Kim D. H., Whittemore S. R., Cao Q. L. (2011) Astrocytes from the contused spinal cord inhibit oligodendrocyte differentiation of adult oligodendrocyte precursor cells by increasing the expression of bone morphogenetic proteins. J. Neurosci. 31, 6053–6058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ben-Hur T., Goldman S. A. (2008) Prospects of cell therapy for disorders of myelin. Ann. N. Y. Acad. Sci. 1142, 218–249 [DOI] [PubMed] [Google Scholar]
- 15. Zawadzka M., Franklin R. J. M. (2007) Myelin regeneration in demyelinating disorders: new developments in biology and clinical pathology. Curr. Opin. Neurol. 20, 294–298 [DOI] [PubMed] [Google Scholar]
- 16. Carbajal K. S., Schaumburg C., Strieter R., Kane J., Lane T. E. (2010) Migration of engrafted neural stem cells is mediated by CXCL12 signaling through CXCR4 in a viral model of multiple sclerosis. Proc. Natl. Acad. Sci. 107, 11068–11073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cushing M. C., Anseth K. S. (2007) Materials science: hydrogel cell cultures. Science 316, 1133–1134 [DOI] [PubMed] [Google Scholar]
- 18. Discher D. E., Mooney D. J., Zandstra P. W. (2009) Growth factors, matrices, and forces combine and control stem cells. Science 324, 1673–1677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mammoto A., Connor K. M., Mammoto T., Yung C. W., Huh D., Aderman C. M., Mostoslavsky G., Smith L. E. H., Ingber D. E. (2009) A mechanosensitive transcriptional mechanism that controls angiogenesis. Nature 457, 1103–1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tibbitt M. W., Anseth K. S. (2009) Hydrogels as extracellular matrix mimics for 3D cell culture. Bio/Technol. Bioengin. 103, 655–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Marklein R. A., Burdick J. A. (2010) Controlling stem cell fate with material design. Adv. Mat. 12, 175–189 [DOI] [PubMed] [Google Scholar]
- 22. Liu Z., Wang H., Wang Y., Lin Q., Yao A., Cao F., Li D., Zhou J., Duan C., Du Z., Wang Y., Wang C. (2012) The influence of chitosan hydrogel on stem cell engraftment, survival and homing in the ischemic myocardial microenvironment. Biomaterials 33, 3093–3106 [DOI] [PubMed] [Google Scholar]
- 23. Habib M., Shapira-Schweitzer K., Caspi O., Gepstein A., Arbel G., Aronson D., Seliktar D., Gepstein L. (2011) A combined cell therapy and in-situ tissue-engineering approach for myocardial repair. Biomaterials 32, 7514–7523 [DOI] [PubMed] [Google Scholar]
- 24. Ghosh K., Shu X. Z., Mou R., Lombardi J., Prestwich G. D., Rafailovich M. H., Clark R. A. F. (2005) Rheological Characterization of in Situ Cross-Linkable Hyaluronan Hydrogels. Biomacromolecules 6, 2857–2865 [DOI] [PubMed] [Google Scholar]
- 25. Boudou T., Ohayon J., Picart C., Tracqui P. (2006) An extended relationship for the characterization of Young's modulus and Poisson's ratio of tunable polyacrylamide gels. Biorheology 43, 721–728 [PubMed] [Google Scholar]
- 26. Louis J. C., Magal E., Muir D., Manthorpe M., Varon S. (1992) CG-4, A new bipotential glial cell line from rat brain, is capable of differentiating in vitro into either mature oligodendrocytes or type-2 astrocytes. J. Neurosci Res. 31, 193–204 [DOI] [PubMed] [Google Scholar]
- 27. Woodruff R. H., Franklin R. J. M. (1999) Demyelination and remyelination of the caudal cerebellar peduncle of adult rats following stereotaxic injections of lysolecithin, ethidium bromide, and complement/anti-galactocerebroside: A comparative study. Glia 25, 216–228 [DOI] [PubMed] [Google Scholar]
- 28. Lu Y.-B., Franze K., Seifert G., Steinhäuser C., Kirchhoff F., Wolburg H., Guck J., Janmey P., Wei E.-Q., Käs J., Reichenbach A. (2006) Viscoelastic properties of individual glial cells and neurons in the CNS. Proc. Natl. Acad. Sci. 103, 17759–17764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mahoney M. J., Anseth K. S. (2006) Three-dimensional growth and function of neural tissue in degradable polyethylene glycol hydrogels. Biomaterials 27, 2265–2274 [DOI] [PubMed] [Google Scholar]
- 30. Sim F. J., Lang J. K., Waldau B., Roy N. S., Schwartz T. E., Pilcher W. H., Chandross K. J., Natesan S., Merrill J. E., Goldman S. A. (2006) Complementary patterns of gene expression by human oligodendrocyte progenitors and their environment predict determinants of progenitor maintenance and differentiation. Ann. Neurol. 59, 763–779 [DOI] [PubMed] [Google Scholar]
- 31. Carbajal K. S., Miranda J. L., Tsukamoto M. R., Lane T. E. (2011) CXCR4 signaling regulates remyelination by endogenous oligodendrocyte progenitor cells in a viral model of demyelination. Glia 59, 1813–1821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Discher D. E., Janmey P., Wang Y.-l. (2005) Tissue cells feel and respond to the stiffness of their substrate. Science 310, 1139–1143 [DOI] [PubMed] [Google Scholar]
- 33. Hansoo P., Johnna S. T., Yasuhiko T., Arnold I. C., Robert M. R., John A. J., Antonios G. M. (2009) Effect of dual growth factor delivery on chondrogenic differentiation of rabbit marrow mesenchymal stem cells encapsulated in injectable hydrogel composites. J. Biomed. Mater. Res. A 88A, 889–897 [DOI] [PubMed] [Google Scholar]
- 34. Zhong J., Chan A., Morad L., Kornblum H. I., Fan G., Carmichael S. T. (2010) Hydrogel matrix to support stem cell survival after brain transplantation in stroke. Neurorehabil. Neural Repair 24, 636–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Uemura M., Refaat M. M., Shinoyama M., Hayashi H., Hashimoto N., Takahashi J. (2010) Matrigel supports survival and neuronal differentiation of grafted embryonic stem cell-derived neural precursor cells. J. Neurosci. Res. 88, 542–551 [DOI] [PubMed] [Google Scholar]
- 36. Tysseling-Mattiace V. M., Sahni V., Niece K. L., Birch D., Czeisler C., Fehlings M. G., Stupp S. I., Kessler J. A. (2008) Self-assembling nanofibers inhibit glial scar formation and promote axon elongation after spinal cord injury. J. Neurosci. 28, 3814–3823 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.