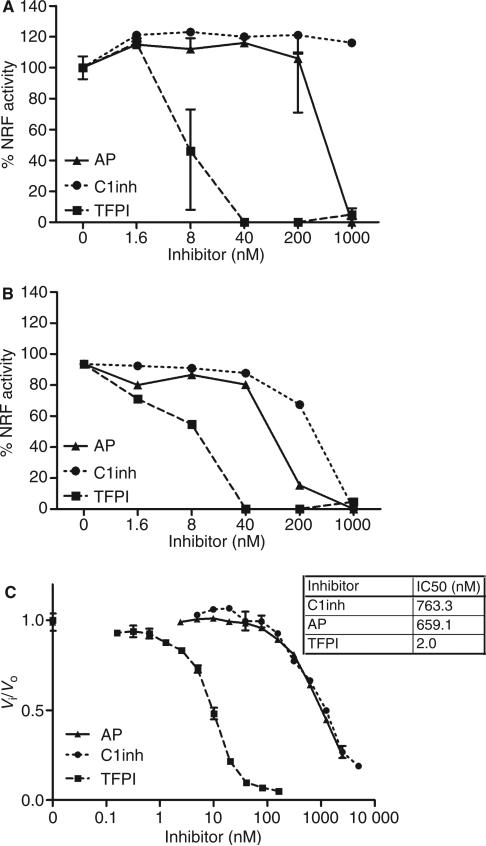
Fig. 1.
Inhibition of factor VII-activating protease (FSAP) activity by α2-antiplasmin (AP), C1-inhibitor (C1inh), and tissue factor pathway inhibitor (TFPI). Serum 10% (A) or purified FSAP 10 AU mL–1 (B) was preincubated with increasing concentrations of AP, C1inh or TFPI prior to incubation with apoptotic cells for 30 min at 37 °C. Cells were stained with propidium iodide, and median fluorescence intensity (MFI) was measured by fluorescence-activated cell sorting analysis. Nucleosome-releasing factor (NRF) activity is expressed as percentage of the decrease in MFI after incubation with 10% serum (which contains ~ 18.5 nm FSAP) or 10 AU mL–1 purified two-chain FSAP (tcFSAP) (~ 18.5 nm). Increasing concentrations of TFPI, C1inh and AP were added to the chromogenic substrate S2288 (1 mm). Purified tcFSAP (10 nm) was added, and the absorbance at 405 nm was measured (C). Data are plotted as Vi/Vo against the inhibitor concentration. Vi and Vo indicate the velocities of substrate hydrolysis by 10 nm tcFSAP in the presence and absence of inhibitor, respectively. The potency in inhibiting 50% of the reference amidolytic activity (IC50) was determined. Results are given as mean ± standard error of the mean (n = 3).