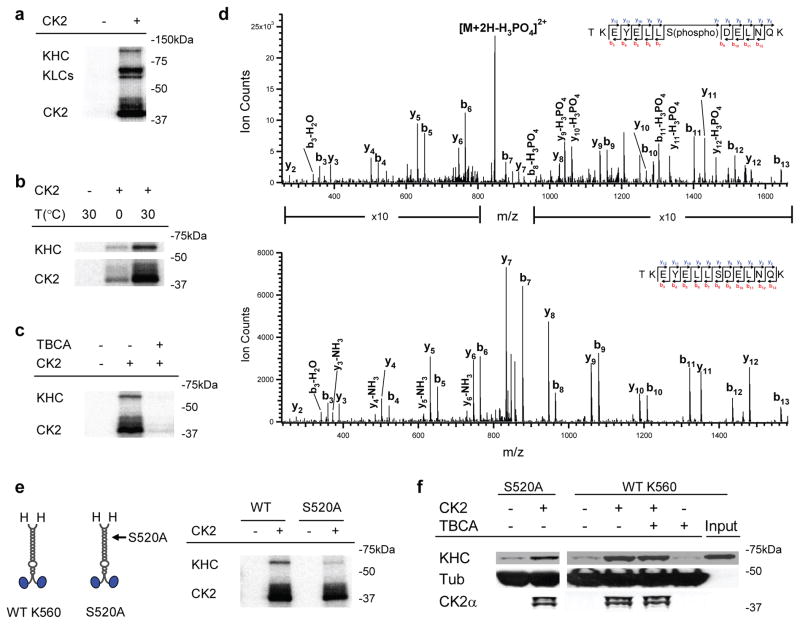
Figure 3.
Role of CK2 kinase activity in K560 activation. (a) Autoradiogram of CK2/native kinesin mixture, supplemented with 1 μCi of [γ-32P]-ATP. Control experiments lacking CK2 are shown. (b) Effect of incubation temperature on CK2 kinase activity in vitro, assayed by autoradiogram. Parallel samples of wild type K560/CK2 mixture, supplemented with 1 μCi of [γ-32P]-ATP, were incubated on ice (1.5 hr) or at 30°C (40 min). Radioactive phosphate incorporation in both K560 and the CK2 itself were severely limited when incubated on ice. (c) Effect of the CK2 specific inhibitor, TBCA (100 μM), on the kinase’s ability to phosphorylate wild type K560, assayed by autoradiogram. Diminished CK2 auto-phosphorylation (in ‘CK2’ band) by TBCA-treatment further verified the pharmacological inhibition of kinase activity. (d) LC-MS/MS spectra of tryptic phosphopeptide (TKEYELLS(phospho)DELNQK) and non-phosphopeptide (TKEYELLSDELNQK). Ion count intensity in the phosphopeptide spectrum is enhanced in the indicated regions for comparison with the non-phosphopeptide. We obtain 81% sequence coverage of K560 protein using LC-MS/MS analysis. (e) Schematic (left) of wild-type K560and phospho-mutant S52A, and autoradiogram (right) assaying the ability of CK2 to phosphorylate the mutant (‘S520A’) vs. wild type motor (‘WT’). (f) Motor co-sedimentation with microtubules at 4 mM AMPPNP, assayed by immunoblot. Both the phosphor-mutant (‘S520A’) and the wild-type motor (‘WT K560’) were incubated with and without CK2 (3:1 CK2:motor) for 40 min at 30 °C prior to microtubule pulldowns. For ‘WT K560’, we introduced the CK2 specific kinase inhibitor, TBCA (100μM), during motor/kinase incubation.