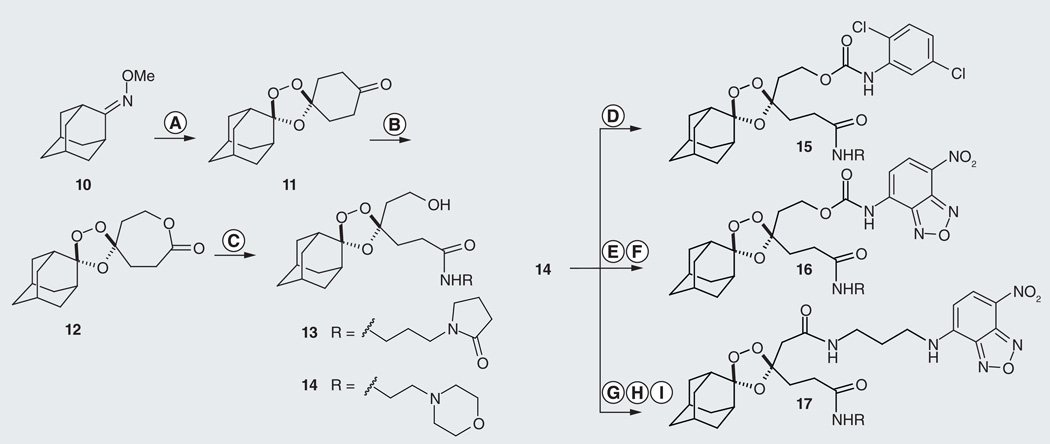
Figure 5. Synthesis of prototypical fragmenting hybrid species 15–17.
Hybrid 15 bears an inactive surrogate for partner drug while analogs 16 and 17 are conditionally and constitutively fluorescent, respectively. Conditions: (A) O3, 1,4-cyclohexadione, pentane, CCl4, 0°C, 1 h, 30–40%; (B) mCPBA, NaHCO3, CH2Cl2, 48 h, 60%; (C) N-3-(aminopropyl)-2-pyrrolidinone (for 13), toluene, 50°C, 95%; or 4-(2-aminoethyl)-morpholine (for 14), toluene, 50°C, 70%; (D) Ar-NCO, toluene, 18 h, 87%; (E) 4-nitrophenylchloroformate, Et3N, DMAP, CH2Cl2, 10 h, 70%; (F) Ar-NH2, DMAP, CH2Cl2, 18 h, 47%; (G) Dess-Martin, CH2Cl2; (H) NaClO2, Na2HPO4, isobutylene, t-BuOH-water-THF, 0°C, 58% over two steps; (I) N-(7-nitro-[1,2,5]benzoxadiazol-4-yl)propane-1,3-diamine, EDC, HOBt, DIEA, DMF, 55%.