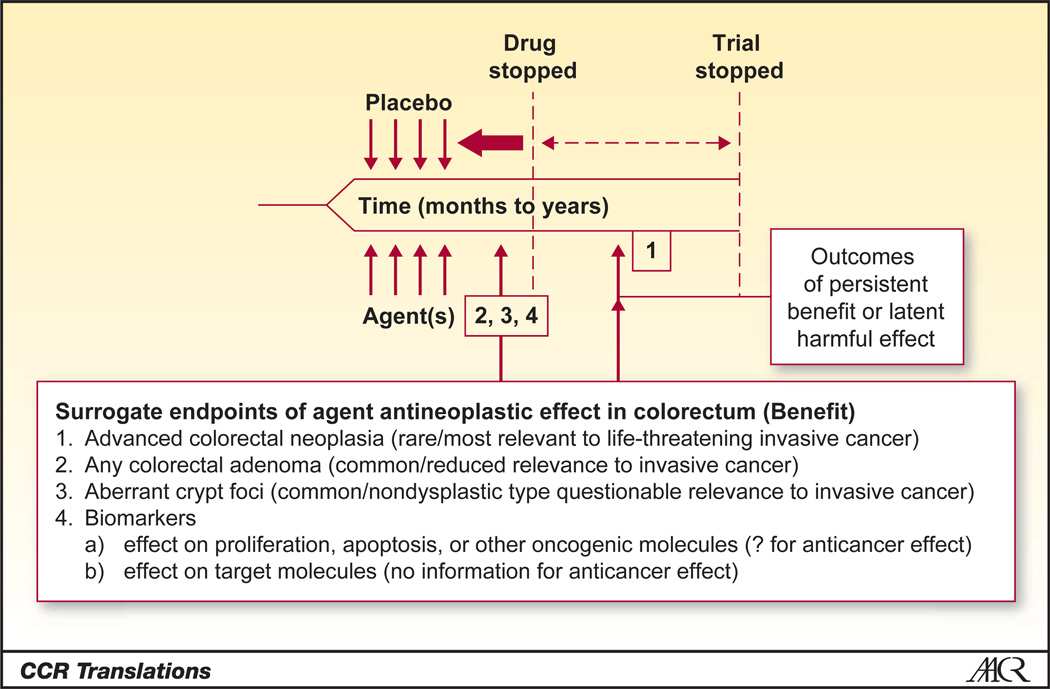
Figure 1.
Fast-tracking the timeline for evaluation of chemoprevention studies of NSAIDs. Integration of the common surrogate endpoints of ACF or any polyp allows design considerations for short interventions with shorter time to follow-up. Truncation of this timeline in an ongoing evaluation of NSAIDs for prevention would allow the formal inclusion of a postintervention evaluation to determine the persistency of effect or emergence of latent harmful effects, aiding in the informed delivery of NSAIDs for chemoprevention. Adapted from Levin, Potential Pitfalls in the Use of Surrogate Endpoints in Colorectal Adenoma Chemoprevention, Journal of the National Cancer Institute, 2003, vol. 95, issue 10, pp. 697–699 by permission of Oxford University Press.