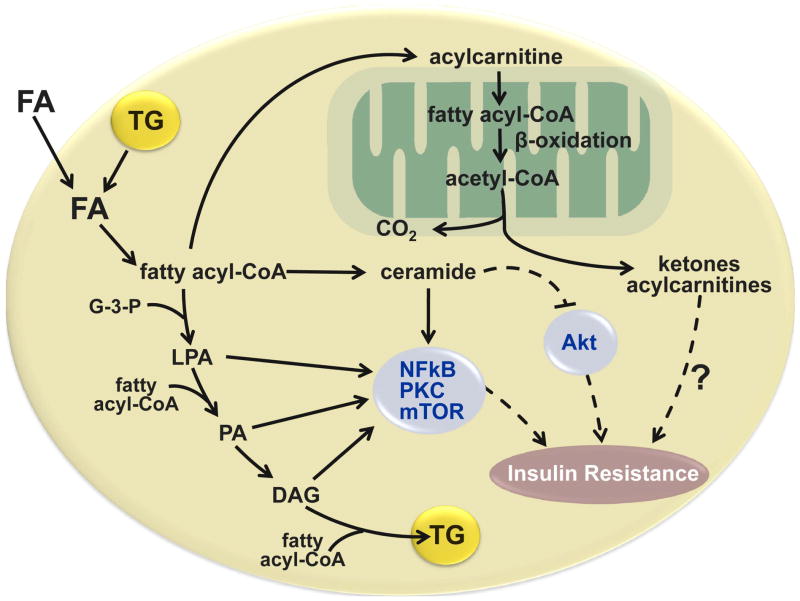
Figure 6.
Potential cellular mechanisms responsible for the relationship between fatty acid metabolism and insulin resistance in the liver and skeletal muscle. Obese persons with nonalcoholic fatty liver disease have increased rates of adipose tissue lipolysis and fatty acid (FA) release into plasma and increased intrahepatic and intramyocellular triglyceride (TG) content. Intracellular FA delivered from plasma or derived from lipolysis of intracellular triglyceride (TG) can be transported to the mitochondria for oxidation, esterified to TG or partially metabolized to several lipid intermediates, long chain fatty acyl-CoA, ceramide, lysophosphatidic acid (LPA), and phosphatidic acid (PA), and diacylglycerol (DAG). These lipid intermediates can interfere with insulin signaling by activating protein kinase C (PKC), mammalian target of rapamycin (mTOR), and nuclear factor kinase B (NFκB), and inhibiting Akt (also known as protein kinase B). The oxidation of intracellular FAs involve the conversion of long-chain fatty acyl-CoAs to acylcarnitines, which enter the mitochondria, and are progressively shortened by β-oxidation, which produces acetyl-CoA that can enter the tricarboxylic acid cycle for complete oxidation. The incomplete oxidation of fatty acyl-CoA generates ketone bodies and acylcarnitines, which might also have adverse effects on insulin action. (Adapted from: Schenk S, Saberi M, Olefsky JM. Insulin sensitivity: modulation by nutrients and inflammation. J Clin Invest 2008;118:2992–3002, and Nagle CA, Klett EL, Coleman RA. Hepatic triacylglycerol accumulation and insulin resistance. J Lipid Res 2009;50 Suppl:S74–79).