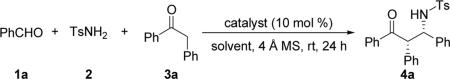
Table 1.
Catalyst screening and condition optimizations for the base-catalyzed three-component direct Mannich reactiona
| entry | catalyst | solvent | yield (%)b | drc | ee (%)d |
|---|---|---|---|---|---|
| 1 | 5 | toluene | 10 | >99:1 | 45 |
| 2 | 6 | toluene | trace | --- | --- |
| 3 | 7 | toluene | 44 | >99:1 | 60 |
| 4 | 8 | toluene | 49 | >99:1 | 78e |
| 5 | 9 | toluene | 96 | >99:1 | 94 |
| 6 | 10 | toluene | 96 | >99:1 | 90e |
| 7 | 11 | toluene | 78 | >99:1 | 94 |
| 8 | 12 | toluene | 68 | >99:1 | 92e |
| 9 | 9 | benzene | 96 | >99:1 | 92 |
| 10 | 9 | o-xylene | 95 | >99:1 | 92 |
| 11 | 9 | CH2Cl2 | 95 | >99:1 | 86 |
| 12 | 9 | Et2O | 87 | >99:1 | 72 |
| 13 | 9 | THF | 60 | >99:1 | 50 |
| 14f | 9 | toluene | 96 | >99:1 | 96 |
Unless otherwise specified, all reactions were carried out at rt with benzaldehyde (1a, 0.20 mmol), toluenesulfonamide (2, 0.40 mmol), ketone 3a (0.40 mmol), and the Lewis base catalyst (0.020 mmol, 10 mol %) in the presence of 4 Å MS (50.0 mg) in the indicated solvent (2 mL) for 24 h.
Yield of the isolated product after column chromatography.
Determined by 1H NMR analysis of the crude reaction product.
Values of ee were determined by chiral HPLC analysis on a ChiralCel AD-H column.
The opposite enantiomer was obtained as the major product in this case.
The reaction was conducted at 0 °C for 48 h.
