Table 2.
Three-component direct Mannich reaction catalyzed by bifunctional catalysts 9 or 10a
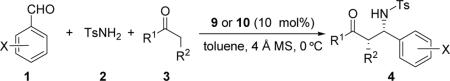
| entry | X/ R1 / R2 | 1/3/4 | time (h) | yield (%)b | ee (%)c |
|---|---|---|---|---|---|
| 1 | H/Ph/Ph | a/a/a | 48 | 96 | 96 |
| 2 | 4-Me/Ph/Ph | b/a/b | 40 | 95 | 96 |
| 3 | 4-MeO/Ph/Ph | c/a/c | 48 | 88 | 96 |
| 4 | 4-F/Ph/Ph | d/a/d | 36 | 96 | 95 |
| 5 | 4-Cl/Ph/Ph | e/a/e | 24 | 95 | 94 |
| 6 | 4-Br/Ph/Ph | f/a/f | 24 | 97 | 94 |
| 7 | 4-CN/Ph/Ph | g/a/g | 40 | 97 | 97 |
| 8 | 4-NO2/Ph/Ph | h/a/h | 24 | 93 | 93 |
| 9 | 2-Br/Ph/Ph | i/a/i | 48 | 96 | 95 |
| 10 | 3-Br/Ph/Ph | j/a/j | 24 | 96 | 95 |
| 11 | H/4-BrC6H4/Ph | a/b/k | 30 | 95 | 98 |
| 12 | H/4-ClC6H4/Ph | a/c/l | 35 | 95 | 96 |
| 13 | H/4-MeOC6H4/Ph | a/d/m | 72 | 75 | 96 |
| 14 | H/Et/Ph | a/e/n | 96 | 75 | >99 |
| 15 | H/PhCH2/Ph | a/f/o | 24 | 95 | 96 |
| 16 | H/Ph/OBoc | a/g/p | 24 | 96d | >99 |
| 17 | 4-Br/Ph/OBoc | f/g/q | 40 | 96e | >99 |
| 18f | H/4-MeOC6H4/OBoc | a/h/r | 48 | 95g | >99 |
Unless otherwise indicated, all reactions were carried out at 0 °C with aldehyde 1 (0.20 mmol), p-toluenesulfonamide (2, 0.40 mmol), ketone 3 (0.40 mmol), catalyst 9 (0.020 mmol, 10 mol %), and 4 Å MS (50. 0 mg) in toluene (2.0 mL).
Yield of the isolated product after column chromatography. Only a single diastereomer was detected by the 1H NMR analysis of the crude reaction product in all cases (dr >99:1) except for entries 16–18.
Determined by HPLC analysis.
The dr of this reaction was 93:7.
The dr of this reaction was 92:8.
Catalyst 10 (10 mol %) was used, and the opposite enantiomer was obtained as the major product.
The dr of this reaction was 90:10.
