Figure 6. The TRPP3 coiled-coil domain trimer is important for the assembly and surface expression of homomeric TRPP3 complexes.
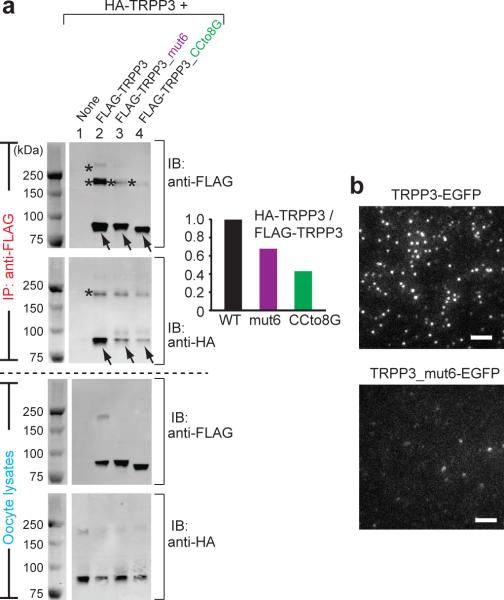
(a) HA- and FLAG-tagged full-length TRPP3, as indicated on top, were expressed in Xenopus oocytes and immunoprecipitated (IP) with anti-FLAG antibody coated beads. All proteins had comparable expression as can be seen from the lysate samples (lower two gels). Reduced amount of HA-tagged TRPP3 was co-IPed with FLAG-tagged TRPP3 mutants that lack the coiled-coil trimer interaction (upper gel, lanes 3 and 4). IB: immunoblot. On SDS-PAGE, WT TRPP3 gave rise to a monomeric band at ~90 kDa (arrows) and two oligomeric bands with molecular masses of ~200 kDa and >250 kDa (asterisks). The oligomeric complexes appeared even at more severe denaturing and reducing conditions (Supplementary Fig. S8). The two FLAG-tagged TRPP3 mutants showed reduced amount of both oligomeric bands, suggesting a weakened homomeric assembly of TRPP3 (upper gel immunobloted with anti-Flag antibody, lanes 3 and 4; also see Fig. 7c, d). Bar graph at right shows the normalized ratio of the intensity of HA-TRPP3 (WT or mutants as indicated at bottom)/Flag-TRPP3. The sum of the intensity of all monomeric and oligomeric bands for each TRPP3 protein was used in the calculation. A smaller ratio suggests a weakened association between HA-TRPP3 and Flag-TRPP3. (b) TIRF images of EGFP fluorescence from oocytes expressing the indicated constructs. Upper panel was a duplication of Fig. 3a. Scale bar, 2 μm.
(Yang)