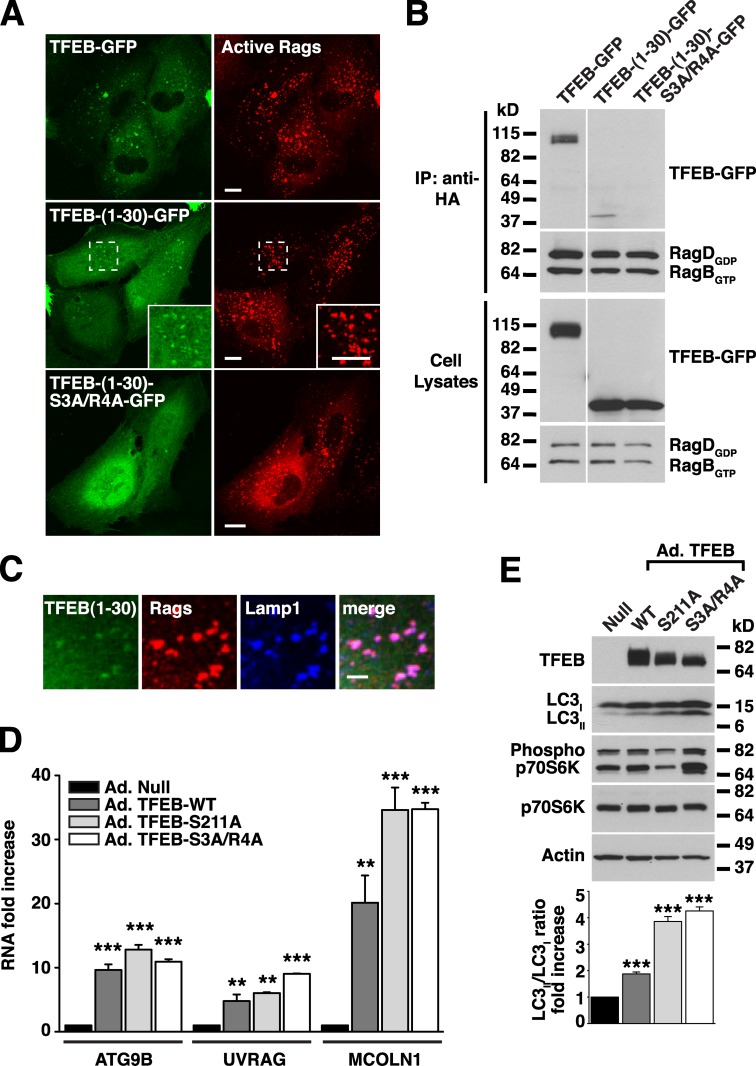
Figure 7.
The first 30 amino acids of TFEB are sufficient for binding to active Rag heterodimers. (A) ARPE-19 cells coexpressing active RagB/D heterodimer and the indicated TFEB plasmids were fixed, permeabilized with 0.2% saponin, and stained with antibodies against GST (used to detect Rag proteins). Regions within the dotted boxes are magnified in the insets. Bars, 10 µm. (B) ARPE-19 cells were cotransfected with active RagB/D heterodimers and the indicated TFEB constructs. After 18 h, cell lysates were immunoprecipitated with the anti-HA antibody (used to immunoprecipitate Rag proteins) and immunoblotted with antibodies against GFP and GST (used to detect TFEB-GFP and Rag proteins, respectively). The white line indicates that intervening lanes have been spliced out. (C) Immunofluorescence confocal microscopy showing the subcellular distribution of TFEB-(1–30)-GFP in ARPE-19 cells coexpressing active RagB/D heterodimers (antibodies against GST were used to detect Rags). Bar, 4 µm. (D) Relative RT-PCR analysis of the mRNA expression of autophagy (ATG9B and UVRAG) and lysosomal (MCOLN1) genes in ARPE-19 cells infected with the indicated adenovirus for 48 h. The values are expressed as a ratio to RNA from cells infected with control adenovirus (Ad.-Null). Values are means ± SD of two independent experiments. ***, P < 0.001; **, P < 0.01. (E) Cells were infected with adenovirus as in D and analyzed by immunoblotting with the indicated antibodies. IP, immunoprecipitation.