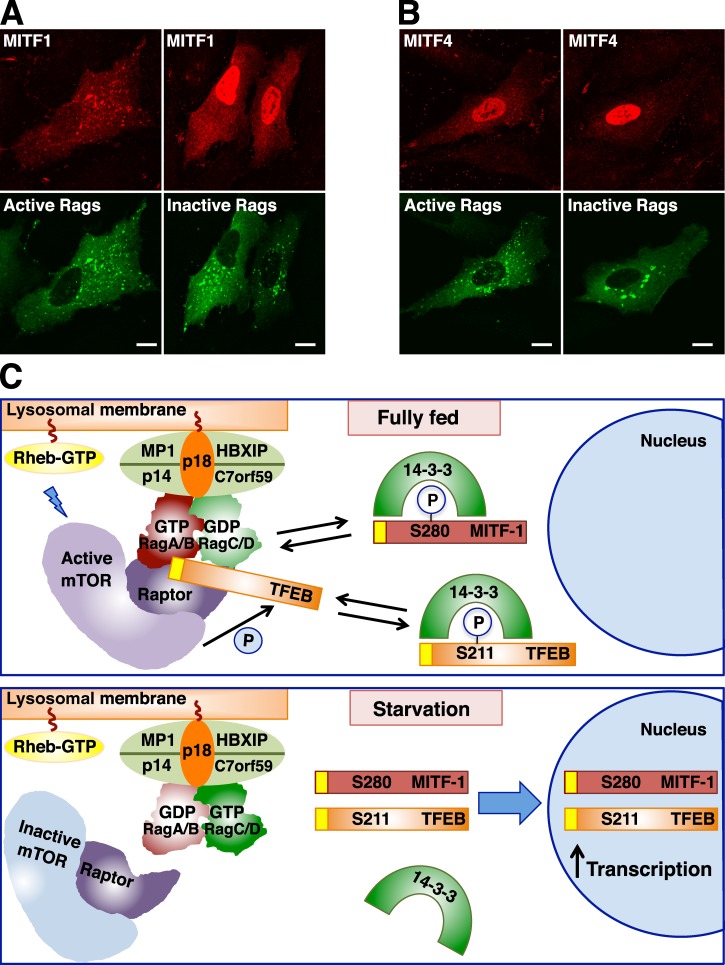
Figure 9.
Rag GTPases regulate recruitment of MITF and TFEB to lysosomes. (A and B) ARPE-19 cells were nucleofected with the indicated Rag- and MITF-expressing plasmids. 12 h later, cells were fixed, permeabilized with 0.2% Triton X-100, and double stained with antibodies against FLAG and GST (used to detect MITF and Rag proteins, respectively). Bars, 10 µm. (C) Model representing the mechanism of TFEB and MITF regulation by Rag GTPases. (top) In nutrient-rich conditions, active Rags promote recruitment of mTORC1 and TFEB to lysosomes, thus facilitating mTORC1-dependent phosphorylation of TFEB. Phosphorylation of TFEB at S211 creates a binding site for 14-3-3 and results in sequestration of TFEB in the cytosol. We suggest that 14-3-3 may mask the Rag-binding domain in TFEB (represented in yellow). (bottom) In the absence of amino acids, Rag GTPases and mTORC1 are inactivated. Dissociation of the TFEB–14-3-3 complex allows transport of TFEB to the nucleus and TFEB-mediated activation of a transcriptional network that promotes autophagy, lysosomal biogenesis, and increased lysosomal degradation. Our model proposes that some MITF isoforms might be regulated in a similar manner. The interaction of MITF-1 with 14-3-3 has been previously described (Bronisz et al., 2006). Please note that mTORC1-dependent phosphorylation of MITF-1 S280 has not been reported and is merely speculative. The representation of the Ragulator–Rag–mTORC1 complex is based on the recent crystal structured described by Gong et al. (2011). P, phosphorylation.