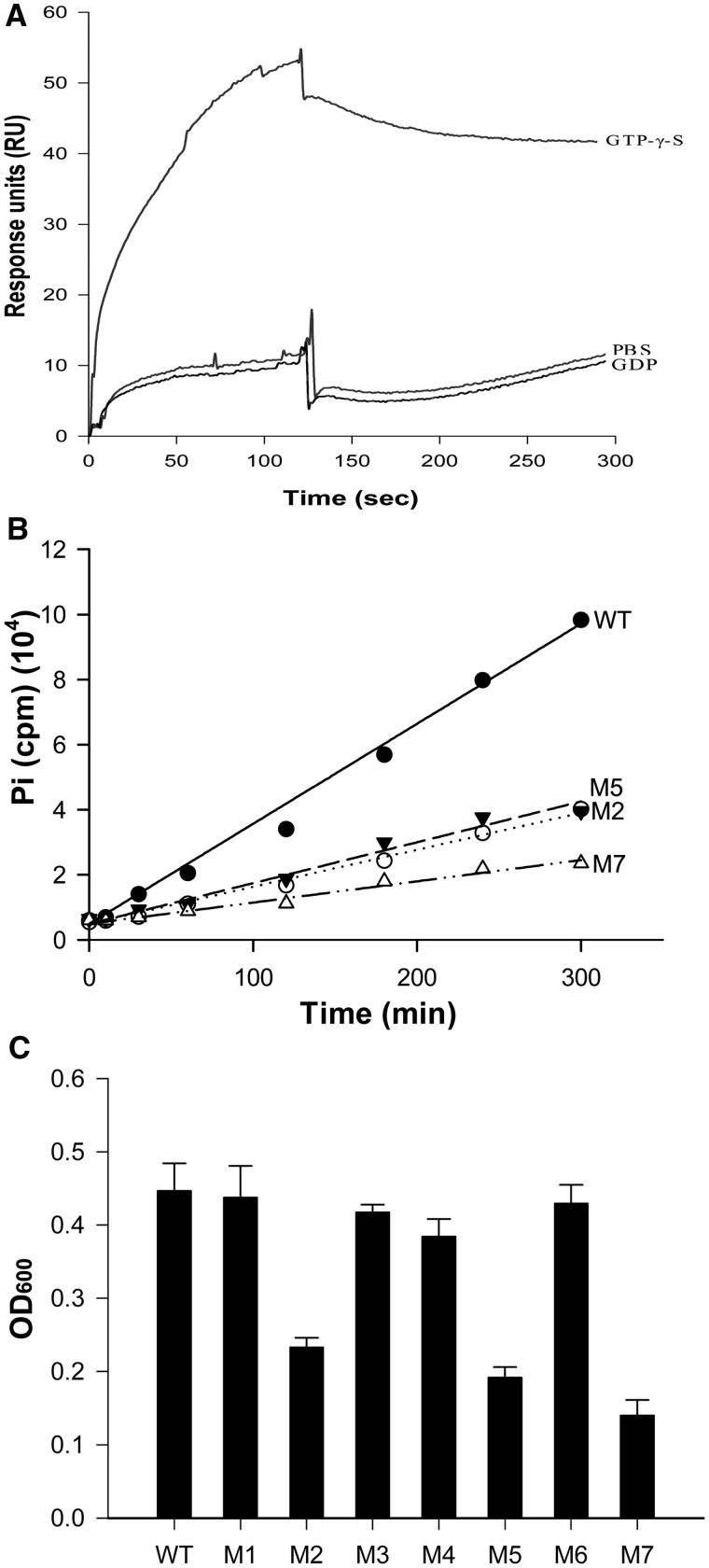
Figure 5.
The GTPase activity of MfpB is indispensable for protection from CIP interference. (A) GTP-bound MfpB interacts directly with MfpA. SPR was used to determine the binding affinity of GDP-MfpB and GTP-MfpB to MfpA. Purified MfpA-His6 was coated directly on CM5 biosensor chips, then 10 μM purified GTP-γ-S or GDP-bound or nucleotide-free MfpB was injected. (B) MfpB mutants with mutated conserved GTPase domain residues have correspondingly reduced GTPase activities. In all, 4 μM of purified proteins were incubated with 15 μM of [γ-32P]GTP at 37°C, and 50 μl of samples were collected at the times indicated. Hydrolysis of GTP was measured by 32Pi release as described in Figure 4B. (C) GTPase-deficient mutants had decreased growth rates in the presence of 0.25 µg/ml of CIP. Drug resistance was measured as described in Figure 1B.