Abstract
XPC/Rad4 (human/yeast) recruits transcription faction IIH (TFIIH) to the nucleotide excision repair (NER) complex through interactions with its p62/Tfb1 and XPB/Ssl2 subunits. TFIIH then recruits XPG/Rad2 through interactions with similar subunits and the two repair factors appear to be mutually exclusive within the NER complex. Here, we show that Rad4 binds the PH domain of the Tfb1 (Tfb1PH) with high affinity. Structural characterization of a Rad4–Tfb1PH complex demonstrates that the Rad4-binding interface is formed using a motif similar to one used by Rad2 to bind Tfb1PH. In vivo studies in yeast demonstrate that the N-terminal Tfb1-binding motif and C-terminal TFIIH-binding motif of Rad4 are both crucial for survival following exposure to UV irradiation. Together, these results support the hypothesis that XPG/Rad2 displaces XPC/Rad4 from the repair complex in part through interactions with the Tfb1/p62 subunit of TFIIH. The Rad4–Tfb1PH structure also provides detailed information regarding, not only the interplay of TFIIH recruitment to the NER, but also links the role of TFIIH in NER and transcription.
INTRODUCTION
Exposure to ultraviolet (UV) irradiation can threaten the survival of organisms due to its ability to damage DNA by inducing modifications such as cyclobutane pyrimidine dimers (CPD) and (6,4) photoproducts (6,4PP). Due to the constant threat that UV irradiation poses, organisms have developed pathways like nucleotide excision repair (NER) to repair the damaged DNA (1). Failure to repair the damage ultimately threatens the integrity of the organism’s genome, and it is now clear that defects in factors that function in the NER repair pathway results in a number of debilitating human diseases including xeroderma pigmentosum (XP) (2). XP is an autosomal recessive genetic disorder characterized by a hypersensitivity to UV irradiation, and the most common human form of XP is associated with mutations of the complementation group XP-C (3). XP patients are defective in their ability to perform NER following exposure to UV irradiation, which results in an increased probability of acquiring a number of different forms of skin cancers (4).
NER can be divided into two pathways depending on whether the damaged DNA that needs to be repaired is being actively transcribed (transcription-coupled NER; TC-NER) or not (global genome NER; GG-NER). Although these two pathways of NER share many similarities, they differ considerably in how the damaged DNA is initially recognized. In GG-NER, the XPC-RAD23B/Rad4-Rad23 (human/yeast) complex recognizes the damaged DNA, sometimes with the help of UV-DDB (UV-damaged DNA-binding factor) (5,6). In contrast, during TC-NER, the RNA polymerase II (RNAP II) complex stalls on the damaged site, which leads to the recruitment of the Cockayne syndrome B (CSB/Rad26) protein (7,8). Following this initial recognition of the damaged DNA site, the second step of both NER pathways involves the recruitment of the general transcription faction IIH (TFIIH) due to the presence of either CSB/Rad26 (TC-NER) or XPC/Rad4 (GG-NER) (7–11).
As a crucial component of the GG-NER pathway, XPC/Rad4 participates in a number of protein–protein and protein–DNA interactions. Studies with the full-length XPC/Rad4 demonstrated that the central region of Rad4 (residues 120–630) was sufficient for both specific binding to the CPD-damaged DNA and heterodimerization with Rad23 (12). These studies also concluded that the amino-terminal (N-terminal; residues 1–120) and carboxyl-terminal (C-terminal; residues 630–754) regions of Rad4 are most likely disordered based on their sensitivity to proteolysis and this is consistent with nuclear magnetic resonance (NMR) studies showing that the C-terminal 125 amino acids of XPC (residues 816–940) are also disordered (13). Despite being disordered, several studies have shown that the N- and C-terminal regions of XPC/Rad4 play important roles in interactions with Centrin-2, Cdc31, Rad33 and TFIIH (9,14–17).
TFIIH is a 10-subunit complex that is highly conserved between humans and budding yeast (Saccharomyces cerevisiae). Once recruited to the damaged DNA site by XPC/Rad4, TFIIH plays several important functions. First, through the helicase activities of its XPB/Ssl2 and XPD/Rad3 subunits, TFIIH assists in unwinding the DNA in and around the damaged site (18). Next, TFIIH recruits the 3′-endonuclease XPG/Rad2, which initiates the cutting of the DNA and the subsequent excision/repair processes (19). In GG-NER, the recruitment of XPG/Rad2 to the damaged DNA site coincides with the release of the XPC-RAD23B/Rad4–Rad23 complex, and this exchange between XPC/Rad4 and XPG/Rad2 appears to be mediated through interactions involving TFIIH (10,20,21). As a result, a number of studies have examined the interaction between TFIIH and XPC/Rad4 to understand its role in NER. Co-immunoprecipitation (Co-IP) studies with Rad4 indicated that residues 1–167 are sufficient for binding to TFIIH (9). In addition, in vitro and in vivo studies showed that both the XPB and p62 subunits of TFIIH directly interact with XPC and that the C-terminal region of XPC plays an important role in recruiting the TFIIH complex (17,22). Interestingly, the two subunits of TFIIH that interact with XPC/Rad4 (p62/Tfb1 and XPB/Ssl2) are also involved in recruiting XPG/Rad2 (23,24), and this suggests these two repair factors may form similar types of interactions with TFIIH that lead to their exchange during NER.
Despite the important role that the XPC/Rad4–TFIIH complex plays in regulating GG-NER, there is currently no detailed information describing the regions of XPC/Rad4 that interact with either the p62/Tfb1 or XPB/Ssl2 subunits of TFIIH. In this study, we characterize the interaction between Rad4 and the Tfb1 subunit of TFIIH. We identify an acidic segment from the N-terminal region of Rad4 (Rad476–115; residues 76–115) that binds with high affinity to the PH domain located at the N-terminus of the Tfb1 subunit of TFIIH (Tfb1PH; residues 1–115). NMR spectroscopy studies show that Rad476–115 binds to the same region of Tfb1PH as two homologous acidic segments from Rad2, and Rad476–115 competes with these Rad2 segments for binding to Tfb1PH. Structural characterization of a Rad476–115–Tfb1PH complex indicates that Rad4 binds to Tfb1PH using a very similar Tfb1PH-binding motif as Rad2 (25). In addition, in vivo studies show that yeast with mutations of key residues of Rad4 required for interaction with Tfb1 display enhanced sensitivity to UV irradiation. Together these results provide a rationale for the role that TFIIH binding plays in the exchange between XPC/Rad4 and XPG/Rad2 during GG-NER.
MATERIALS AND METHODS
Strains, media and vectors
Saccharomyces cerevisiae strains used for these studies are listed in Supplementary Table S1. The strains were grown a synthetic complete media (SC: 0.67% yeast nitrogen base without amino acids, 2% glucose and a mixture of amino acids and vitamins) lacking uracil (SC-U) for selection. All yeast transformations were performed using the modified lithium acetate protocol (26).
Plasmid preparations
The pRS316RAD4cmyc plasmid (RAD4) was generated by amplification of the RAD4 open reading frame (ORF) complemented by 190-bp upstream and 282-bp downstream on genomic DNA and insertion into pRS316. The pRS316RAD4(F95P/V98P)cmyc plasmid (rad4-PP) was obtained by using QuikChange II site-directed mutagenesis kit (Stratagene). The pRS316RAD4(W649A/L652A/L656A)cmyc (rad4-AAA) and pRS316RAD4(F95P/V98P/W649A/L652A/L656A)cmyc (rad4-PPAAA) plasmids were both obtained by overlapped PCR using either pRS316RAD4cmyc or pRS316RAD4(F95P/V98P)cmyc, respectively, as the cloning vector (For details see ‘Supplementary Methods’ section).
Sensitivity assays
Yeast strains were grown overnight at 30°C in selective media. The next day they were diluted in order to obtain an OD595 = 0.5–1 the following morning. The cells were then harvested by centrifugation, washed and resuspended in sterile water to obtain an OD595 = 0.5. For UV-sensitivity assays, dilutions were plated on selective media (SC-U) and irradiated with UV light (XL-1000 UV crosslinker, SpectroLinker) at varying energy levels. The surviving colonies were counted after 3 days growth at 30°C, in the dark.
Cloning and purification of proteins
The GST-Tfb1PH (residues 1–115 of Tfb1) was prepared as described (27). GST-Rad476–115 was prepared by inserting the appropriate region of Rad4 (Open Biosystems) into the pGEX-2T expression vector. GST-Rad3441–63 was prepared by inserting the appropriate region of Rad34 (Open Biosystems) into the pGEX-2T expression vector. All point mutants were made using QuikChange II site-directed mutagenesis kit (Stratagene). All coding sequences were verified by DNA sequencing. Tfb1PH was purified as described (27). Rad476–115, GST-Rad3441–63 and their mutants were expressed as GST-fusion proteins in Escherichia coli host strain TOPP2, purified over GSH resin (GE Healthcare) and cleaved with thrombin (Calbiochem), as previously described for Tfb1PH (27). Following cleavage with thrombin, the proteins were purified over a Q-Sepharose High Performance (GE Healthcare) column and dialyzed into appropriate buffers for isothermal titration calorimetry (ITC) and NMR studies. 15N-labeled and 15N/13C-labeled proteins were prepared in M9-minimal media containing 15NH4Cl (Sigma) and/or 13C6-glucose (Sigma), as the sole nitrogen and carbon sources. For all experiments, protein concentrations were determined from A280.
ITC experiments
ITC titrations were performed as described (28) at 25°C in 20 mM sodium phosphate buffer (pH 7.5). All titrations fit a single-binding site mechanism with 1:1 stoichiometry and values are the average of two or more separate experiments.
NMR experiments
The NMR chemical shift perturbation and competition experiments were performed as previously described (for sample details please see ‘Supplementary Methods’ section). For the NMR structural studies of the Rad476–115–Tfb1PH complex, four different samples containing 1.0 mM of the complex in a 1:1.25 ratio were used (15N-Tfb1PH–Rad476–115, 15N/13C–Tfb1PH-Rad476–115, 15N-Rad476–115–Tfb1PH and 15N/13C-Rad476–115–Tfb1PH, respectively). All NMR experiments were carried out in 20 mM sodium phosphate (pH 6.5), 1 mM ethylenediaminetetraacetic acid, 1 mM DTT and 90% H2O/10% D2O or 100% D2O, at 300 K on Varian Unity Inova 500, 600 and 800 MHz spectrometers equipped with z-pulsed-field gradient units and triple resonance probes. All of the 1H, 15N and 13C resonances for Rad476–115 and Tfb1PH were assigned as reported for free Tfb1PH (29). Briefly, 3D HNCO (30), 3D HNCACB (31), 3D CBCACONH (32), 3D (H)C(CO)NH (33), 3D H(CCO)NH (33) and 3D HCCH–COSY (34) spectra were used to assign the backbone and aliphatic side chains resonances. The aromatic side chains 1H, 13C and 15N resonances were assigned using a combination of 2D (HB)CB(CGCD)HD and 2D (HB)CB(CGCDCE)HE spectra (35). Interproton distance restraints were measured from 3D 15N-edited NOESY-HSQC, 13C-edited HMQC-NOESY (τm = 90 ms) and 3D 15N/13C {F1}-filtered, {F3}-edited NOESY spectra (τm = 90 ms) (36,37). The NMR data were processed with NMRPipe/NMRDraw (38) and analyzed with NMRView (39) and Analysis from the CCPNMR suite (40).
Structure calculations
The nuclear Overhauser effect-derived distance restraints (NOE) were divided. However, other NAR papers use NOE into four classes defined as strong (1.8–2.8 Å), medium (1.8–4.0 Å), weak (1.8–5.0 Å) and very weak (3.3–6.0 Å). Backbone dihedral angles were derived with the program TALOS+ (41). The structure of the Rad476–115–Tfb1PH complex was calculated using the program CNS (42). The quality of the structures was analyzed with the programs PROCHECK-NMR (43) and MOLMOL (44). Ramachandran plot analysis showed that 76.9, 20.7, 1.5 and 0.9% of residues are in the most favored, additionally allowed, generously allowed and disallowed regions, respectively. The figures were generated with the program PyMol (Schrödinger).
RESULTS
The N-terminal segment of Rad4 binds Tfb1PH with high affinity
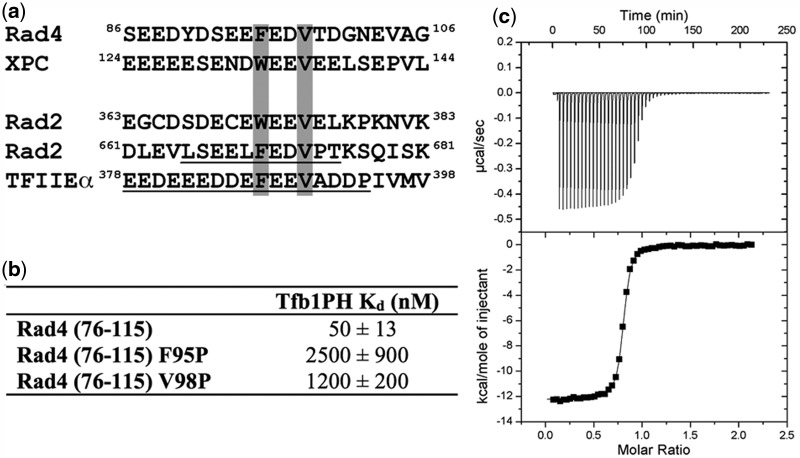
We previously identified two acidic segments within the spacer region of Rad2 (Rad2359–383 and Rad2642–690) that contain a high-affinity Tfb1PH-binding motif (25). Based on the fact that XPC/Rad4 and XPG/Rad2 have been shown to be mutually exclusive in their binding to TFIIH (20,21), we analyzed the sequences of Rad4 and XPC to see if they also contain a region similar to the Tfb1PH-binding motif found in Rad2. The requirements for the two Tfb1PH-binding motifs of Rad2 are that they are located within highly acidic segments and they contain an aromatic residue (W or F) followed by two acidic residues and a valine residue. As shown in Figure 1a, sequences similar to the Tfb1PH-binding motifs of Rad2 are located near the N-terminus of both Rad4 (residues 86–106) and XPC (residues 124–144). This observation is consistent with the first 167 amino acids of Rad4 being sufficient for binding to TFIIH and the fact that the p62/Tfb1 subunit of TFIIH has been shown to directly interact with XPC/Rad4 (9,17,22).
Figure 1.
The N-terminal region of Rad4 contains a high-affinity Tfb1PH-binding site. (a) Identification of an amino acid segment located between residues 86 and 106 of Rad4 that aligns with the Tfb1PH-binding motif found in Rad2 and TFIIEαCTD. A similar motif is also located between residues 124 and 144 of XPC. In these alignments, the residues of TFIIEαCTD and Rad2642–690 that form the binding interface with p62PH/Tfb1PH are underlined and crucial hydrophobic residues are shaded in gray. (b) Comparison of the dissociation constant (Kd) values for the binding of Rad476–115 and its mutants (F95P and V98P) to Tfb1PH. (c) Thermogram of the Tfb1PH titration with successive additions of Rad476–115. Experiments are performed at 25°C, in 20 mM NaPO4 pH 7.5 buffer, and the results fit to a single-binding site model with 1:1 stoichiometry.
To determine whether the N-terminal region of Rad4 does in fact bind to Tfb1PH, the apparent dissociation constant (Kd) between Tfb1PH and Rad476–115 was measured by ITC (Figure 1b and c). As we observed with Rad2359–383 and Rad2642–690 (Kd = 130 nM and Kd = 190 nM, respectively), Rad476–115 binds to Tfb1PH with high affinity (Kd = 50 nM). Next, we wanted to verify that Phe95 and Val98 of Rad4 were crucial residues in the Tfb1PH-binding motif of Rad4. These residues correspond to Phe670 and Val673 of Rad2642–690 and are crucial for Rad2 binding to Tfb1PH (25). Mutation of either Phe95 or Val98 to proline (F95P and V98P) reduces the affinity of Rad476–115 for Tfb1PH by 50- and 24-fold, respectively (Figure 1b), supporting that the Tfb1PH-binding site of Rad4 is very similar to the two sites found in Rad2.
Rad476–115, Rad2359–383 and Rad2642–690 bind a common site on Tfb1PH
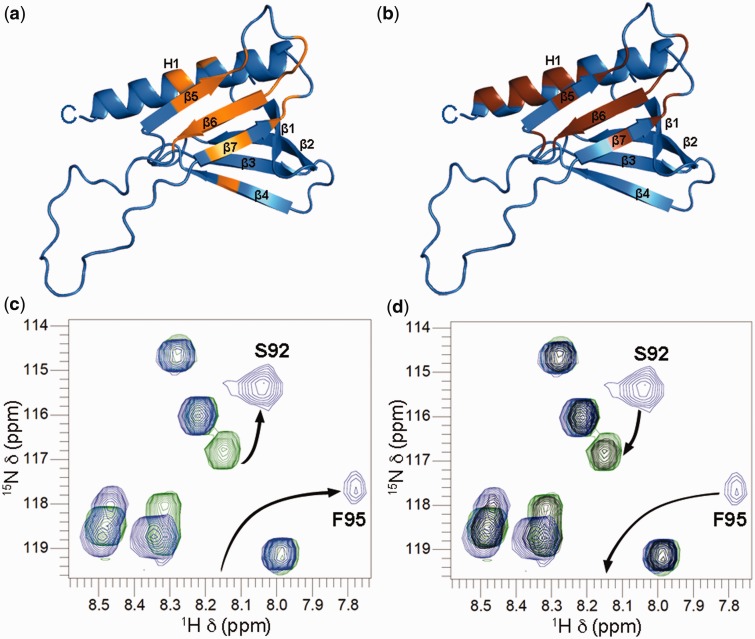
To identify the binding site of Rad476–115 on Tfb1PH, NMR chemical shift perturbation studies were performed. In these experiments, addition of unlabeled Rad476–115 to 15N-labeled Tfb1PH causes significant changes in the 1H and 15N chemical shifts for several Tfb1PH signals in the 1H–15N HSQC spectra (Supplementary Figure S1a and b). When mapped onto the structure of Tfb1PH, the residues exhibiting significant changes are located in strands β5, β6, β7 and helix H1 (Figure 2a). The residues experiencing significant changes are almost identical to those observed when either of the two acidic segments from Rad2 (Rad2359–383 and Rad2642–690) bind to Tfb1PH (Figure 2b and Supplementary Figure S1c).
Figure 2.
Rad476–115 and Rad2359–383 share a common binding site on Tfb1PH. (a and b) Ribbon model of the 3D structure of Tfb1PH (blue; PDB code 1Y5O). The amino acids of Tfb1PH showing a significant chemical shift change {Δδ(ppm) > 0.15; Δδ = [(0.17ΔNH)2 + (ΔHN)2]1/2} upon formation of a complex with either Rad476–115 (a) or Rad2359–383 (b) are highlighted in orange and brown, respectively. (c) Overlay of a selected region from the 1H –15N HSQC spectra of 15N-labeled Rad476–115 (0.5 mM) in the free form (green) and in the presence of unlabeled Tfb1PH (0.4 mM; blue). (d) Same overlay as in (c), but after the addition of unlabeled Rad2359–383 (1.5 mM; black). Rad476–115 signals that undergo significant changes in 1H and 15N chemical shifts upon formation of the complex with Tfb1PH (c), and return towards their original position following the addition of Rad2359–383 (d) are indicated by arrows.
To confirm that Rad476–115 shares a common binding site on Tfb1PH with Rad2359–383, NMR competition experiments were performed. In these experiments, we first add a substoichiometric concentration of unlabeled Tfb1PH (0.4 mM) to a sample containing 15N-labeled Rad476–115 (0.5 mM) and as expected, we observe significant changes for several 1H and 15N chemical shifts in the 1H–15N HSQC spectra of Rad476–115 (Figure 2c). We then add an equimolar amount of unlabeled Rad2359–383 (0.5 mM) to the 15N-Rad476–115 –Tfb1PH complex and observe that the 1H and 15N resonances of Rad476–115, which shift upon formation of the Rad476–115–Tfb1PH complex return to values characteristic of the free form of Rad476–115 (Figure 2d). Taken together with previous results showing that Rad2359–383 and Rad2642–690 compete for binding to Tfb1PH (25), these results demonstrate that Rad476–115, Rad2359–383 and Rad2642–690 all compete for a common binding site on Tfb1PH.
The Rad4 homolog Rad34 also contains a Tfb1PH-binding motif
Rad34 is a repair factor found in yeast that plays a role similar to Rad4 in TC-NER of ribosomal DNA (rDNA) (45,46). Given the fact that TFIIH also plays a critical role in RNA polymerase I (RNAP I)-dependent transcription and TC-NER of rDNA (47), we were interested to determine whether Rad34 also contained a Tfb1PH-binding motif. The Rad34 protein is homologous to the Rad4 protein, and it contains both a DNA-binding domain that recognizes damaged DNA and a TFIIH/Centrin-2-binding motif within its C-terminal region (48). Although there is currently no evidence that Rad34 interacts with TFIIH, we postulated based on its homology to Rad4, that it also contains a Tfb1PH-binding motif near the N-terminus of the protein. Based on sequence homology with Rad475–116, we identified a segment between residues 41 and 63 of Rad34 (Rad3441–63; Figure 3a) as a potential Tfb1PH-binding motif, since it contains a highly acidic stretch that includes an aromatic residue (Trp54) followed by two acidic residues and a valine (Val57). ITC studies show that Rad3441–63 also binds to Tfb1PH with high affinity (Kd = 11.0 nM; Figure 3b) and that mutation of Trp54 to a serine (W54S mutant) significantly reduces binding to Tfb1PH (over three orders of magnitude). To further define the mode of binding of Rad3441–63 to Tfb1PH, an NMR chemical shift perturbation study was performed. Like for Rad476–115, addition of Rad3441–63 to 15N–Tfb1PH causes significant changes in the 1H and 15N chemical shifts for several Tfb1PH signals in the 1H–15N HSQC spectra (Supplementary Figure S2) and the residues exhibiting significant changes are located in strands β5, β6, β7 and the H1 helix (Figure 3c).
Figure 3.
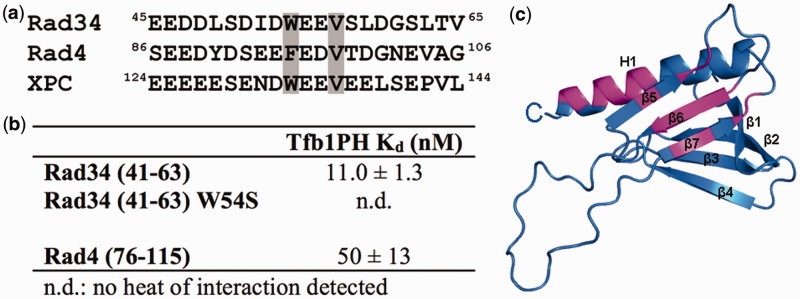
Rad34 contains a Tfb1PH-binding motif. (a) Identification of an amino acid segment located between residues 41 and 63 from Rad34 that aligns with the Tfb1PH-binding motif from Rad4 and XPC. The two crucial hydrophobic residues in the motif are shaded in gray. (b) Comparison of the dissociation constant (Kd) values for the binding of Rad476–115 and Rad3441–63 with Tfb1PH. No binding is observed with the W54S mutant of Rad3441–63 under the experimental conditions indicating a Kd > 10 µM. (c) Ribbon model of the 3D structure of Tfb1PH (blue). The amino acids of 15N-labeled Tfb1PH showing a significant chemical shift change {Δδ(ppm) > 0.15; Δδ = [(0.17ΔNH)2 + (ΔHN)2]1/2} upon formation of a complex with Rad3441–63 are highlighted in magenta.
The two TFIIH-binding regions of Rad4 are crucial to survival following UV irradiation
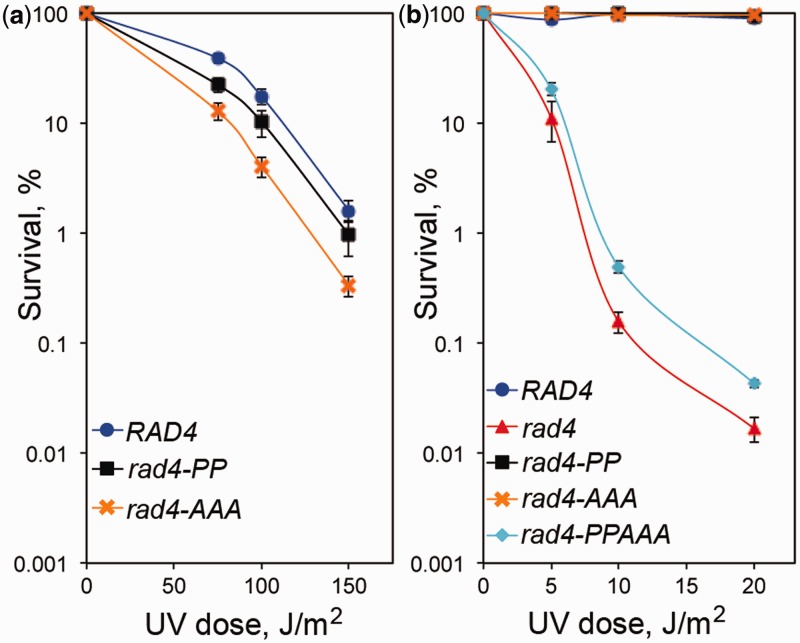
Previous studies have shown that both the N- and C-terminal regions of XPC/Rad4 interact with TFIIH (9,14–17). To examine the functional role of these two TFIIH-binding regions of Rad4 in yeast survival following exposure to UV irradiation, we first prepared a yeast strain in which the two key hydrophobic residues (Phe95 and Val98) in the Tfb1PH-binding motif were mutated to proline (rad4-PP) and we tested it for survival following exposure to UV irradiation. The rad4-PP strain shows only a slightly enhanced sensitivity to UV irradiation when compared with the wild-type RAD4 strain (Figure 4a). Next, we tested the role of the C-terminal TFIIH-binding motif of Rad4. Previous studies have shown that mutating three key hydrophobic residues (Trp649, Leu652 and Leu656) in this region to alanine leads to yeast strains (rad4-AAA) with enhanced sensitivity to UV irradiation as well as decreased binding to TFIIH and Centrin-2 (15). Consistent with previous studies, the rad4-AAA mutant strain is more sensitive to UV irradiation than the RAD4 strain as well as slightly more sensitive than the rad4-PP strain (Figure 4a).
Figure 4.
The two TFIIH-binding regions of Rad4 are crucial for survival following UV irradiation. (a) The survival of RAD4 (blue), rad4-PP (black) and rad4-AAA yeast were determined following increasing doses of UV irradiation. (b) The survival of RAD4 (blue), rad4 (red), rad4-PP (black), rad4-AAA (orange) and rad4-PPAAA (aqua) yeast were determined following increasing doses of UV irradiation. In both (a) and (b), the y-axis represents the percentage of surviving cells (normalized to the number of viable cells not exposed to UV light) and the x-axis shows the energy levels of the UV irradiation applied (J/m2). The results are the mean ± SEM of three independent experiments.
To further examine the in vivo role of the two TFIIH-binding regions of Rad4, the N-terminal mutation (rad4-PP) and the C-terminal mutation (rad4-AAA) were combined to produce an additional mutant strain (rad4-PPAAA). The survival curves show that the rad4-PPAAA strain is considerably more sensitive to UV irradiation than either the rad4-PP or rad4-AAA strains and displays similar sensitivity to what is observed with the rad4 deletion strain (Figure 4b). This increased sensitivity to UV irradiation is not the result of decreased levels of Rad4 as the Rad4-PPAAA mutant protein is expressed at similar levels as the wild-type Rad4 (Supplementary Figure S3). Taken together, the in vivo studies in yeast indicate that the Tfb1PH-binding motif of Rad4 plays a key role in yeast survival following exposure to UV irradiation. In addition, mutating both of the known TFIIH-binding regions of Rad4 produces a dramatic effect on yeast sensitivity to UV irradiation, suggesting that at least two distinct interactions occur between Rad4 and TFIIH and that these interactions appear to be almost redundant for Rad4 function in DNA repair.
NMR structure determination of the Rad476–115–Tfb1PH complex
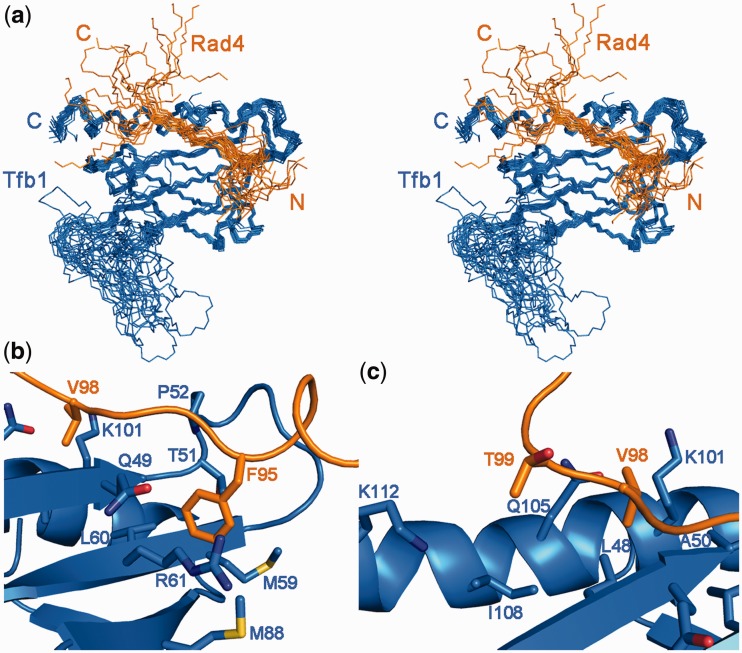
To compare Rad2 and Rad4 in complex with Tfb1PH, we determined the NMR solution structure of a Rad476–115–Tfb1PH complex. The structure of the Rad476–115–Tfb1PH complex (PDB code 2M14) is well defined by the NMR data (Table 1). The 20 lowest energy structures (Figure 5a) are characterized by good backbone geometry, no significant restraint violation and low pair-wise r.m.s.d. values (Table 1). As is the case for the Rad2642–690–Tfb1PH complex, the structure of Tfb1PH in complex with Rad476–115 is virtually identical to its free form showing a typical PH domain fold consisting of a seven-stranded β-sandwich (β1–β7) flanked on one side by a long α-helix (H1) (27). In complex with Tfb1PH, Rad476–115 binds in an extended conformation devoid of any regular secondary structural element with residues 95–98 forming a crucial portion of the interface with Tfb1PH. This is consistent with the 1H–15N HSQC spectra of the titration of 15N-labeled Rad476–115 with Tfb1PH as residues 95–98 undergo the most significant changes in both their 1H and 15N chemical shifts (Supplementary Figure S4).
Table 1.
NMR and refinement statistics for Rad4 in complex with Tfb1PHa
| NMR distance and dihedral constraints | |
| Distance constraints | |
| Total NOE | 1599 |
| Intra-residue | 556 |
| Inter-residue | |
| Sequential (|i – j| = 1) | 359 |
| Medium-range (|i – j| < 4) | 200 |
| Long-range (|i – j| > 5) | 484 |
| Intermolecular | 28 |
| Hydrogen bonds | 36 |
| Total dihedral angle restraints | 148 |
| ϕ | 74 |
| ψ | 74 |
| Structure statistics | |
| Violations (mean and SD) | |
| Distance constraints (Å) | 0.0179 ± 0.0006 |
| Dihedral angle constraints (°) | 0.18 ± 0.03 |
| Max. dihedral angle violation (°) | 5 |
| Max. distance constraint violation (Å) | 0.5 |
| Deviations from idealized geometry | |
| Bond lengths (Å) | 0.00210 ± 0.00007 |
| Bond angles (°) | 0.376 ± 0.004 |
| Impropers (°) | 0.209 ± 0.008 |
| Average pairwise r.m.s. deviation (Å)b | |
| Heavy | 1.31 ± 0.12 |
| Backbone | 0.67 ± 0.14 |
aThe 20 conformers with the lowest energies were selected for statistical analysis.
bOnly residues 5–63 and 86–112 of Tfb1PH and residues 95–99 of Rad4 were used for the r.m.s.d. calculations. Residues at the N-terminus (1–4), at the C-terminus (113–115), and in the flexible loop (64–85) of Tfb1PH, as well as residues at the N-terminus (76–94) and at the C-terminus (100–115) of Rad4 were not included in the calculation.
Figure 5.
NMR structure of the Rad476–115–Tfb1PH complex. (a) Stereo view of the 20 lowest-energy structures of the complex between Tfb1PH (blue) and Rad476–115 (orange; PDB code 2M14). The 3D structures were superimposed using the backbone atoms C′, Cα and N of residues 4–65 and 85–112 of Tfb1PH and residues 90–104 of Rad476–115. (b) Ribbon representation of Tfb1PH (blue) and backbone trace of the region of Rad476–115 (orange) interacting in the first binding pocket. In this pocket, Phe95 of Rad4 forms a cation–π interaction with Arg61 of Tfb1 and van der Waals interactions with Met59. (c) Ribbon representation of Tfb1PH (blue) and backbone trace of the region of Rad476–115 (orange) interacting in the second binding pocket. On one side of the pocket Val98 of Rad4 interacts with Leu48, Ala50, Lys101 and Gln105 of Tfb1. On the other side of the pocket Thr99 of Rad4 interacts with Gln105, Ile108 and Lys112 of Tfb1.
Rad476–115–Tfb1PH-binding interface
In the complex, Rad476–115 binds in an extended form to two adjacent shallow grooves on the surface of Tfb1PH. The first groove is formed by residues Gln49, Ala50, Thr51, Pro52, Met59, Leu60, Arg61 and Met88 from strands β5, β6 and β7 of Tfb1PH (Figure 5b). Phe95 of Rad4 inserts into this groove where it is in position to form a cation–π interaction with Arg61. The second groove is composed of Leu48, Ala50, Lys101 and Gln105, Ile108, Lys112 of Tfb1PH and accommodates Val98 and Thr99 of Rad4 (Figure 5c). Val98 is anchored on one side of this pocket through van der Waals interactions with both its methyl groups. Thr99 is anchored on the other side of the pocket through van der Waals interactions with its methyl group. Although the majority of the interactions within the two grooves are van der Waals contacts, an extensive series of positively charged residues on the surface of Tfb1PH (Lys47, Lys57, Arg61, Arg86, Lys101 and Lys112) surround the two grooves, where they function to position the negatively charged Rad476–115 (Supplementary Figure S5). The NMR structures support the formation of one potential salt bridge between Asp97 of Rad476–115 and Lys47 of Tfb1PH.
Comparison of the Rad476–115–Tfb1PH and Rad2642–690–Tfb1PH interfaces
The structure of the Rad476–115–Tfb1PH interface is virtually identical to the structure of the Rad2642–690–Tfb1PH interface (Figure 6). In both structures, a phenylalanine (Phe95 in Rad4 and Phe670 in Rad2) binds in a first pocket, where it is in position to make a cation–π interaction with Arg61 of Tfb1PH, or the analogous Gln64 in p62PH. In addition, a valine (Val98 in Rad4 and Val673 in Rad2) inserts into a second pocket, where it makes van der Waals contacts with a series of residues in Tfb1PH. Rad4 and Rad2 also contain a threonine (Thr99 in Rad4 and Thr675 in Rad2) that forms van der Waals contacts in the second binding pocket. In Rad2642–690, Val673 and Thr675 are separated by Pro674, whereas in Rad476–115, Val98 and Thr99 are consecutive residues. In Rad2642–690, Pro674 creates a slight bend in the backbone so that the side-chains of Val673 and Thr675 can both be oriented towards the surface of Tfb1PH. One other important commonality between the two structures is that Rad476–115 and Rad2642–690 both contain several acidic residues that participate in electrostatic interactions with basic amino acids that surround the perimeter of the two binding pockets of Tfb1PH. Overall, the similarities between these two interfaces provide a structural framework to understand how Rad4 and Rad2 can compete for binding to Tfb1 in NER.
Figure 6.
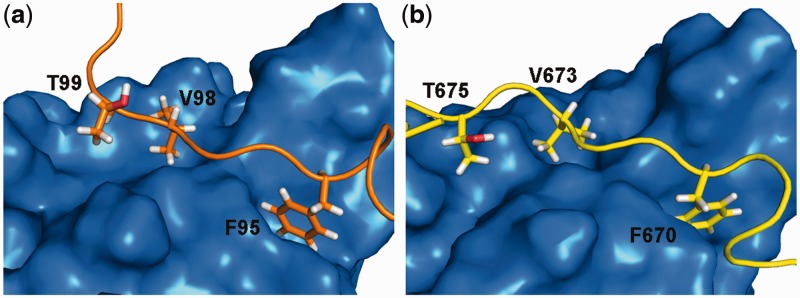
The interfaces of Rad2–Tfb1PH and Rad4–Tfb1PH complexes are very similar. The 3D structures of Tfb1PH are shown as molecular surfaces (blue) and Rad476–115 (a; orange), Rad2642–690 (b; yellow) are shown as ribbons. Selected residues in the Tfb1PH-binding modif of Rad476–115 and Rad2642–690 are also shown to demonstrate the similarity between their binding interfaces.
DISCUSSION
Following DNA damage recognition, the recruitment of TFIIH by XPC/Rad4 to the repair complex occurs through direct interactions with its p62/Tfb1 and XPB/Ssl2 subunits (17,22). Once at the damaged DNA, TFIIH recruits XPG/Rad2 through direct interactions with multiple subunits including both the p62/Tfb1 and XPB/Ssl2 subunits (23,24). Given that XPC/Rad4 and XPG/Rad2 interact with the same subunits of TFIIH and appear to be mutually exclusive within the repair complex (10,20), XPG/Rad2 likely displaces XPC/Rad4 from the pre-incision complex in part by competing for common binding sites on the various subunits of TFIIH (Supplementary Figure S6). Thus, the competition observed for regions of Rad4 and Rad2 binding to Tfb1PH by NMR and the remarkable similarity between the structures of the Rad476–115–Tfb1PH and Rad2642–690–Tfb1PH interfaces provides a molecular mechanism to rationalize the mutual exclusivity observed between XPC/Rad4 and XPG/Rad2 at the repair complex in vivo. Although a hand-off or displacement mechanism has previously been suggested between XPC/Rad4 and XPG/Rad2 in NER (49), this is the first structural evidence to support such a displacement mechanism.
This displacement of XPC/Rad4 by XPG/Rad2 could serve at least two possible purposes for maintaining the NER complex in a repair-competent form. First, it assures that TFIIH is always stabilized on the DNA and available for repair as opposed to being recruited to perform alternative functions such as transcription. Second, it helps to establish the correct orientation of XPG/Rad2 on the DNA lesion. XPG/Rad2 cleaves the phosphate backbone of DNA on the 3′-side of the lesion, and it is crucial that this is done in a precise fashion for NER to be carried out in an accurate manner. Since XPC/Rad4 specifically interacts on the same side of the damaged DNA (12), a sequential displacement mechanism would serve to place XPG/Rad2 in the correct orientation for precise repair of the DNA lesion without the need for release of TFIIH. This model is similar to the ‘passing the baton’ mechanism seen in base excision repair (BER) (50). In the BER model, the substrate is channeled from one protein to another as opposed to a pre-formed protein complex that possesses all the activities necessary for repair.
The fact that both the N-terminal Tfb1PH-binding motif and the C-terminal TFIIH/Centrin-2-binding region of Rad4 play a role in yeast survival following exposure to UV irradiation is consistent with the sequential displacement mechanism between XPC/Rad4 and XPG/Rad2 in NER. In the case of XPG/Rad2, it has also been shown to interact with multiple subunits of TFIIH, including p62/Tfb1 and XPB/Ssl2, by participating in a series of weak interactions with its extended spacer region (23,51,52). Similarly, XPC/Rad4 has been shown to directly interact with both the p62/Tfb1 and XPB/Ssl2 subunits of TFIIH through regions in both termini of the protein (10,20) and our in vivo results in yeast support the importance of both these regions. The fact that a mutation of the C-terminal region of Rad4 has a slightly larger effect on yeast survival following UV irradiation than a mutation of the N-terminal region, is consistent with previous results showing that the C-terminal region of XPC appeared critical for TFIIH binding (17). Taken together with our current results, this indicates that although displacement of both the N-terminal and the C-terminal region of XPC/Rad4 by XGP/Rad2 is important for NER, the displacement of the C-terminal region may be the rate-limiting step. However, the details of the interaction between the C-terminal region of Rad4 and TFIIH are currently unknown and it is still not clear in which order the displacement of the two regions of XPC/Rad4 occurs.
The sequential displacement mechanism clearly presents advantages for maintaining the integrity and accuracy of the repair complex; however, it may also be important for sequestering TFIIH. We have previously shown that the Rad2642–690–Tfb1PH interface is similar to the interface formed in a complex between the C-terminal domain of TFIIEα (TFIIEαCTD) and p62PH (25,53). Like Rad4 and Rad2, TFIIEαCTD contains a Tfb1PH-binding motif that consists of an aromatic residue followed by two acidic residues and a valine (54). We previously suggested that the similarity of the Tfb1PH-binding motif in Rad2 and TFIIEα provides a mechanistic link between the role of TFIIH in transcription and repair. Our current work further strengthens this concept since a highly similar Tfb1PH-binding motif is present in XPC, Rad4 and Rad34. TFIIH plays an important role both in RNAP I-dependent transcription as well as in NER of rDNA (47), and Rad34 likely forms a similar interaction with Tfb1 that may regulate RNAP I-associated NER. Based on the structural similarities of the Rad476–115–Tfb1PH, Rad2642–690–Tfb1PH and the TFIIEαCTD–p62PH interfaces, it is tempting to propose that the interplay of TFIIEα, Rad4 and Rad2 interacting with p62/Tfb1 helps to determine whether TFIIH is functioning in transcription or repair. In RNAP II-dependent transcription, TFIIEαCTD recruits TFIIH to the pre-initiation complex (PIC) through interactions with the p62/Tfb1 subunit of TFIIH (54). Once at the PIC, TFIIH serves multiple functions during transcription by virtue of its helicase (XPB/Ssl2 and XPD/Rad3) and kinase (Cdk7/Kin28) activities (55,56). During NER, TFIIH’s role in transcription would be limited by its recruitment to the DNA lesion through interactions with Rad4 that involves in part the same p62/Tfb1 subunit. As repair progresses past the initial recognition of the DNA lesion to the incision steps, TFIIH remains associated with the repair complex through interactions with XPG/Rad2. This would make the process more efficient since it would help to prevent TFIIH being recruited for transcription.
In conclusion, we provide the first structural evidence for the displacement mechanism between XPC/Rad4 and XPG/Rad2 during NER. Our data shows that XPC/Rad4 and XPG/Rad2 form similar interactions with the p62/Tfb1 subunit of TFIIH, and this supports the hypothesis that XPG/Rad2 displaces XPC/Rad4 from the repair complex through a series of interactions with different subunits of TFIIH. In addition, the structure of the Rad476–115–Tfb1PH complex provides a common mechanistic explanation for the recruitment of TFIIH to both the PIC and the NER complex. Future studies are needed to determine if other subunits of TFIIH, in particular XPB/Ssl2, also form similar interactions with XPC/Rad4 and XPG/Rad2, and what roles they play in the displacement of XPC/Rad4 by XPG/Rad2.
ACCESSION NUMBERS
2M14.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online: Supplementary Tables 1 and 2, Supplementary Figures 1–6, Supplementary Methods and Supplementary Reference [57].
FUNDING
The Canadian Cancer Society (J.G.O.). J.L.-V. is a Vanier Canada Graduate Scholar from the Canadian Institutes of Health Research. L.C. is a postdoctoral fellow of the Natural Sciences and Engineering Research Council of Canada CREATE program. P.L. is a Canadian Research Chair in Structural Biology and Engineering of RNA. 800 MHz NMR experiments were recorded at the Québec/Eastern Canada High Field NMR Facility, supported by the Natural Sciences and Engineering Research Council of Canada. Funding for open access charge: Canadian Cancer Society.
Conflict of interest statement. None declared.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank Aurélie Bernier and Antoine Chiasson for help with protein purification and Dr Tara Sprules for assistance with several NMR experiments.
REFERENCES
- 1.Friedberg EC, Walker GC, Siede W, Wood RD, Schultz T, Ellenberger T. DNA Repair and Mutagenesis. Washington, DC: ASM Press; 2005. [Google Scholar]
- 2.Lehmann AR. DNA repair-deficient diseases, xeroderma pigmentosum, Cockayne syndrome and trichothiodystrophy. Biochimie. 2003;85:1101–1111. doi: 10.1016/j.biochi.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 3.Cartault F, Nava C, Malbrunot AC, Munier P, Hebert JC, N'Guyen P, Djeridi N, Pariaud P, Pariaud J, Dupuy A, et al. A new XPC gene splicing mutation has lead to the highest worldwide prevalence of xeroderma pigmentosum in black Mahori patients. DNA Repair. 2011;10:577–585. doi: 10.1016/j.dnarep.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 4.Hoeijmakers JH. Genome maintenance mechanisms for preventing cancer. Nature. 2001;411:366–374. doi: 10.1038/35077232. [DOI] [PubMed] [Google Scholar]
- 5.Sugasawa K, Ng JM, Masutani C, Iwai S, van der Spek PJ, Eker AP, Hanaoka F, Bootsma D, Hoeijmakers JH. Xeroderma pigmentosum group C protein complex is the initiator of global genome nucleotide excision repair. Mol. Cell. 1998;2:223–232. doi: 10.1016/s1097-2765(00)80132-x. [DOI] [PubMed] [Google Scholar]
- 6.Fitch ME, Nakajima S, Yasui A, Ford JM. In vivo recruitment of XPC to UV-induced cyclobutane pyrimidine dimers by the DDB2 gene product. J. Biol. Chem. 2003;278:46906–46910. doi: 10.1074/jbc.M307254200. [DOI] [PubMed] [Google Scholar]
- 7.Tantin D, Kansal A, Carey M. Recruitment of the putative transcription-repair coupling factor CSB/ERCC6 to RNA polymerase II elongation complexes. Mol. Cell. Biol. 1997;17:6803–6814. doi: 10.1128/mcb.17.12.6803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sarker AH, Tsutakawa SE, Kostek S, Ng C, Shin DS, Peris M, Campeau E, Tainer JA, Nogales E, Cooper PK. Recognition of RNA polymerase II and transcription bubbles by XPG, CSB, and TFIIH: insights for transcription-coupled repair and Cockayne syndrome. Mol. Cell. 2005;20:187–198. doi: 10.1016/j.molcel.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 9.Bardwell AJ, Bardwell L, Iyer N, Svejstrup JQ, Feaver WJ, Kornberg RD, Friedberg EC. Yeast nucleotide excision repair proteins Rad2 and Rad4 interact with RNA polymerase II basal transcription factor b (TFIIH) Mol. Cell. Biol. 1994;14:3569–3576. doi: 10.1128/mcb.14.6.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Araujo SJ, Nigg EA, Wood RD. Strong functional interactions of TFIIH with XPC and XPG in human DNA nucleotide excision repair, without a preassembled repairosome. Mol. Cell. Biol. 2001;21:2281–2291. doi: 10.1128/MCB.21.7.2281-2291.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yokoi M, Masutani C, Maekawa T, Sugasawa K, Ohkuma Y, Hanaoka F. The xeroderma pigmentosum group C protein complex XPC-HR23B plays an important role in the recruitment of transcription factor IIH to damaged DNA. J. Biol. Chem. 2000;275:9870–9875. doi: 10.1074/jbc.275.13.9870. [DOI] [PubMed] [Google Scholar]
- 12.Min J-H, Pavletich NP. Recognition of DNA damage by the Rad4 nucleotide excision repair protein. Nature. 2007;449:570–575. doi: 10.1038/nature06155. [DOI] [PubMed] [Google Scholar]
- 13.Miron S, Duchambon P, Blouquit Y, Durand D, Craescu CT. The carboxy-terminal domain of xeroderma pigmentosum complementation group C protein, involved in TFIIH and centrin binding, is highly disordered. Biochemistry. 2008;47:1403–1413. doi: 10.1021/bi701863u. [DOI] [PubMed] [Google Scholar]
- 14.Chen L, Madura K. Centrin/Cdc31 is a novel regulator of protein degradation. Mol. Cell. Biol. 2008;28:1829–1840. doi: 10.1128/MCB.01256-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.den Dulk B, van Eijk P, de Ruijter M, Brandsma JA, Brouwer J. The NER protein Rad33 shows functional homology to human Centrin2 and is involved in modification of Rad4. DNA Repair. 2008;7:858–868. doi: 10.1016/j.dnarep.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 16.Popescu A, Miron S, Blouquit Y, Duchambon P, Christova P, Craescu CT. Xeroderma pigmentosum group C protein possesses a high affinity binding site to human centrin 2 and calmodulin. J. Biol. Chem. 2003;278:40252–40261. doi: 10.1074/jbc.M302546200. [DOI] [PubMed] [Google Scholar]
- 17.Uchida A, Sugasawa K, Masutani C, Dohmae N, Araki M, Yokoi M, Ohkuma Y, Hanaoka F. The carboxy-terminal domain of the XPC protein plays a crucial role in nucleotide excision repair through interactions with transcription factor IIH. DNA Repair. 2002;1:449–461. doi: 10.1016/s1568-7864(02)00031-9. [DOI] [PubMed] [Google Scholar]
- 18.Coin F, Oksenych V, Egly J-M. Distinct roles for the XPB/p52 and XPD/p44 subcomplexes of TFIIH in damaged DNA opening during nucleotide excision repair. Mol. Cell. 2007;26:245–256. doi: 10.1016/j.molcel.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 19.Schärer OD. The molecular basis for different disease states caused by mutations in TFIIH and XPG. DNA Repair. 2008;7:339–344. doi: 10.1016/j.dnarep.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Riedl T, Hanaoka F, Egly JM. The comings and goings of nucleotide excision repair factors on damaged DNA. EMBO J. 2003;22:5293–5303. doi: 10.1093/emboj/cdg489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wakasugi M, Sancar A. Assembly, subunit composition, and footprint of human DNA repair excision nuclease. Proc. Natl Acad. Sci. USA. 1998;95:6669–6674. doi: 10.1073/pnas.95.12.6669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bernardes de Jesus BM, Bjoras M, Coin F, Egly JM. Dissection of the molecular defects caused by pathogenic mutations in the DNA repair factor XPC. Mol. Cell. Biol. 2008;28:7225–7235. doi: 10.1128/MCB.00781-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hohl M, Dunand-Sauthier I, Staresincic L, Jaquier-Gubler P, Thorel F, Modesti M, Clarkson SG, Schärer OD. Domain swapping between FEN-1 and XPG defines regions in XPG that mediate nucleotide excision repair activity and substrate specificity. Nucleic Acids Res. 2007;35:3053–3063. doi: 10.1093/nar/gkm092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iyer N, Reagan MS, Wu KJ, Canagarajah B, Friedberg EC. Interactions involving the human RNA polymerase II transcription/nucleotide excision repair complex TFIIH, the nucleotide excision repair protein XPG, and Cockayne syndrome group B (CSB) protein. Biochemistry. 1996;35:2157–2167. doi: 10.1021/bi9524124. [DOI] [PubMed] [Google Scholar]
- 25.Lafrance-Vanasse J, Arseneault G, Cappadocia L, Chen HT, Legault P, Omichinski JG. Structural and functional characterization of interactions involving the Tfb1 subunit of TFIIH and the NER factor Rad2. Nucleic Acids Res. 2012;40:5739–5750. doi: 10.1093/nar/gks194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaiser C, Michaelis S, Mitchell A. Methods in Yeast Genetics. Cold Spring Harbor, NY: Cold Spring Harbor Laborator Press; 1994. [Google Scholar]
- 27.Di Lello P, Nguyen BD, Jones TN, Potempa K, Kobor MS, Legault P, Omichinski JG. NMR structure of the amino-terminal domain from the Tfb1 subunit of TFIIH and characterization of its phosphoinositide and VP16 binding sites. Biochemistry. 2005;44:7678–7686. doi: 10.1021/bi050099s. [DOI] [PubMed] [Google Scholar]
- 28.Houtman JC, Higashimoto Y, Dimasi N, Cho S, Yamaguchi H, Bowden B, Regan C, Malchiodi EL, Mariuzza R, Schuck P, et al. Binding specificity of multiprotein signaling complexes is determined by both cooperative interactions and affinity preferences. Biochemistry. 2004;43:4170–4178. doi: 10.1021/bi0357311. [DOI] [PubMed] [Google Scholar]
- 29.Nguyen BD, Di Lello P, Legault P, Omichinski JG. 1H, 15N, and 13C resonance assignment of the amino-terminal domain of the Tfb1 subunit of yeast TFIIH. J. Biomol. NMR. 2005;31:173–174. doi: 10.1007/s10858-004-7266-0. [DOI] [PubMed] [Google Scholar]
- 30.Kay LE, Xu GY, Yamazaki T. Enhanced-sensitivity triple-resonance spectroscopy with minimal H2O saturation. J. Magn. Reson. A. 1994;109:129–133. [Google Scholar]
- 31.Wittekind M, Mueller L. HNCACB, a high-sensitivity 3D NMR experiment to correlate amide-proton and nitrogen resonances with the alpha- and beta-carbon resonances in proteins. J. Magn. Reson. B. 1993;101:201–205. [Google Scholar]
- 32.Grzesiek S, Bax A. Correlating backbone amide and side chain resonances in larger proteins by multiple relayed triple resonance NMR. J. Am. Chem. Soc. 1992;114:6291–6293. [Google Scholar]
- 33.Logan TM, Olejniczak ET, Xu RX, Fesik SW. Side chain and backbone assignments in isotopically labeled proteins from two heteronuclear triple resonance experiments. FEBS Lett. 1992;314:413–418. doi: 10.1016/0014-5793(92)81517-p. [DOI] [PubMed] [Google Scholar]
- 34.Ikura M, Kay LE, Bax A. Improved three-dimensional 1H-13C-1H correlation spectroscopy of a 13C- labeled protein using constant-time evolution. J. Biomol. NMR. 1991;1:299–304. doi: 10.1007/BF01875522. [DOI] [PubMed] [Google Scholar]
- 35.Yamazaki T, Forman-Kay JD, Kay LE. Two-dimensional NMR experiments for correlating carbon-13 beta and proton delta/epsilon chemical shifts of aromatic residues in 13C-labeled proteins via scalar couplings. J. Am. Chem. Soc. 1993;115:11054–11055. [Google Scholar]
- 36.Zhang O, Kay LE, Olivier JP, Forman-Kay JD. Backbone 1H and 15N resonance assignments of the N-terminal SH3 domain of drk in folded and unfolded states using enhanced-sensitivity pulsed field gradient NMR techniques. J. Biomol. NMR. 1994;4:845–858. doi: 10.1007/BF00398413. [DOI] [PubMed] [Google Scholar]
- 37.Pascal SM, Muhandiram DR, Yamazaki T, Forman-Kay JD, Kay LE. Simultaneous acquisition of 15N- and 13C-edited NOE spectra of proteins dissolved in H2O. J. Magn. Reson. 1994;103:197–201. [Google Scholar]
- 38.Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A. NMRPipe: A multidimensional spectral processing system based on UNIX pipes. J. Biomol. NMR. 1995;6:277–293. doi: 10.1007/BF00197809. [DOI] [PubMed] [Google Scholar]
- 39.Johnson BA. Using NMRView to visualize and analyze the NMR spectra of macromolecules. Methods Mol. Biol. 2004;278:313–352. doi: 10.1385/1-59259-809-9:313. [DOI] [PubMed] [Google Scholar]
- 40.Vranken WF, Boucher W, Stevens TJ, Fogh RH, Pajon A, Llinas M, Ulrich EL, Markley JL, Ionides J, Laue ED. The CCPN data model for NMR spectroscopy: development of a software pipeline. Proteins. 2005;59:687–696. doi: 10.1002/prot.20449. [DOI] [PubMed] [Google Scholar]
- 41.Shen Y, Delaglio F, Cornilescu G, Bax A. TALOS+: a hybrid method for predicting protein backbone torsion angles from NMR chemical shifts. J. Biomol. NMR. 2009;44:213–223. doi: 10.1007/s10858-009-9333-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brunger AT, Adams PD, Clore GM, DeLano WL, Gros P, Grosse-Kunstleve RW, Jiang JS, Kuszewski J, Nilges M, Pannu NS, et al. Crystallography & NMR system: a new software suite for macromolecular structure determination. Acta Crystallogr. D Biol. Crystallogr. 1998;54:905–921. doi: 10.1107/s0907444998003254. [DOI] [PubMed] [Google Scholar]
- 43.Laskowski RA, Rullmannn JA, MacArthur MW, Kaptein R, Thornton JM. AQUA and PROCHECK-NMR: programs for checking the quality of protein structures solved by NMR. J. Biomol. NMR. 1996;8:477–486. doi: 10.1007/BF00228148. [DOI] [PubMed] [Google Scholar]
- 44.Koradi R, Billeter M, Wuthrich K. MOLMOL: a program for display and analysis of macromolecular structures. J. Mol. Graph. 1996;14:51–55. doi: 10.1016/0263-7855(96)00009-4. [DOI] [PubMed] [Google Scholar]
- 45.Tremblay M, Teng Y, Paquette M, Waters R, Conconi A. Complementary roles of yeast Rad4p and Rad34p in nucleotide excision repair of active and inactive rRNA gene chromatin. Mol. Cell. Biol. 2008;28:7504–7513. doi: 10.1128/MCB.00137-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.den Dulk B, Brandsma JA, Brouwer J. The Rad4 homologue YDR314C is essential for strand-specific repair of RNA polymerase I-transcribed rDNA in Saccharomyces cerevisiae. Mol. Microbiol. 2005;56:1518–1526. doi: 10.1111/j.1365-2958.2005.04607.x. [DOI] [PubMed] [Google Scholar]
- 47.Iben S, Tschochner H, Bier M, Hoogstraten D, Hozak P, Egly JM, Grummt I. TFIIH plays an essential role in RNA polymerase I transcription. Cell. 2002;109:297–306. doi: 10.1016/s0092-8674(02)00729-8. [DOI] [PubMed] [Google Scholar]
- 48.den Dulk B, Sun SM, de Ruijter M, Brandsma JA, Brouwer J. Rad33, a new factor involved in nucleotide excision repair in Saccharomyces cerevisiae. DNA Repair. 2006;5:683–692. doi: 10.1016/j.dnarep.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 49.Stauffer ME, Chazin WJ. Structural mechanisms of DNA replication, repair, and recombination. J. Biol. Chem. 2004;279:30915–30918. doi: 10.1074/jbc.R400015200. [DOI] [PubMed] [Google Scholar]
- 50.Wilson SH, Kunkel TA. Passing the baton in base excision repair. Nat. Struct. Biol. 2000;7:176–178. doi: 10.1038/73260. [DOI] [PubMed] [Google Scholar]
- 51.Dunand-Sauthier I, Hohl M, Thorel F, Jaquier-Gubler P, Clarkson SG, Schärer OD. The spacer region of XPG mediates recruitment to nucleotide excision repair complexes and determines substrate specificity. J. Biol. Chem. 2005;280:7030–7037. doi: 10.1074/jbc.M412228200. [DOI] [PubMed] [Google Scholar]
- 52.Thorel F, Constantinou A, Dunand-Sauthier I, Nouspikel T, Lalle P, Raams A, Jaspers NG, Vermeulen W, Shivji MK, Wood RD, et al. Definition of a short region of XPG necessary for TFIIH interaction and stable recruitment to sites of UV damage. Mol. Cell. Biol. 2004;24:10670–10680. doi: 10.1128/MCB.24.24.10670-10680.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Okuda M, Tanaka A, Satoh M, Mizuta S, Takazawa M, Ohkuma Y, Nishimura Y. Structural insight into the TFIIE-TFIIH interaction: TFIIE and p53 share the binding region on TFIIH. EMBO J. 2008;27:1161–1171. doi: 10.1038/emboj.2008.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Di Lello P, Miller Jenkins LM, Mas C, Langlois C, Malitskaya E, Fradet-Turcotte A, Archambault J, Legault P, Omichinski JG. p53 and TFIIEalpha share a common binding site on the Tfb1/p62 subunit of TFIIH. Proc. Natl Acad. Sci. USA. 2008;105:106–111. doi: 10.1073/pnas.0707892105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tirode F, Busso D, Coin F, Egly JM. Reconstitution of the transcription factor TFIIH: assignment of functions for the three enzymatic subunits, XPB, XPD and cdk7. Mol. Cell. 1999;3:87–95. doi: 10.1016/s1097-2765(00)80177-x. [DOI] [PubMed] [Google Scholar]
- 56.Lu H, Zawel L, Fisher L, Egly JM, Reinberg D. Human general transcription factor IIH phosphorylates the C-terminal domain of RNA polymerase II. Nature. 1992;358:641–645. doi: 10.1038/358641a0. [DOI] [PubMed] [Google Scholar]
- 57.Elagoz A, Callejo M, Armstrong J, Rokeach LA. Although calnexin is essential in S. pombe, its highly conserved central domain is dispensable for viability. J. Cell Sci. 1999;112:4449–4460. doi: 10.1242/jcs.112.23.4449. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.