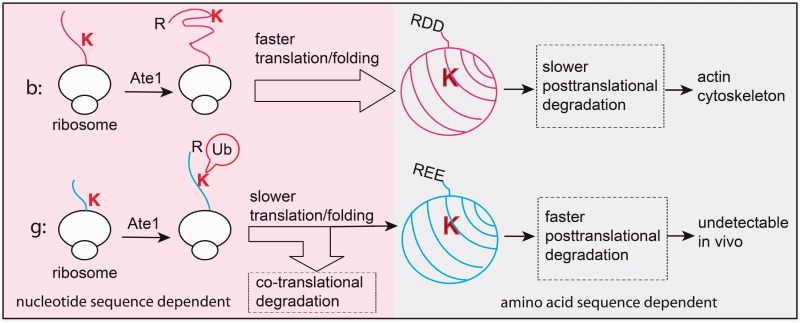
Figure 2.
Differential arginylation of actin isoforms is regulated by a novel degradation mechanism coupled to the translation and folding dynamics in vivo. Top, faster translation and folding of beta-actin protects the Lys18 residue from potential co-translational ubiquitination and degradation on N-terminal arginylation. After emerging from the ribosome, arginylated beta-actin remains relatively stable and incorporates into actin cytoskeleton. Bottom, slower translation and folding of gamma-actin coupled with co-translational arginylation exposes arginylated gamma-actin for ubiquitination and ensures effective removal of 60–80% of arginylated gamma-actin protein. The fraction of arginylated gamma-actin that escapes the co-translational ‘check point’ is still degraded faster, with half-life of only 1 h, so that no arginylated gamma-actin can be detected in vivo. Image courtesy of Dr Fangliang Zhang.