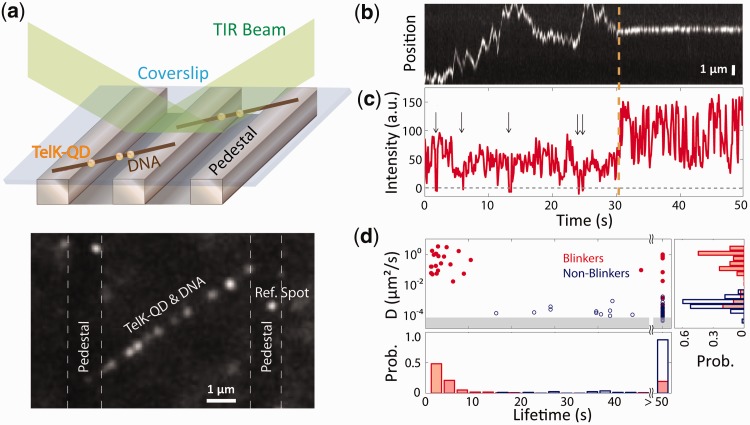
Figure 1.
TelK monomers diffuse along non-target DNA, whereas dimers immobilize. (a) Schematic of TIRFM experimental set-up and representative fluorescence image. An etched glass slide with 1 × 1 µm pedestals separated by 7-µm etches was coated with neutravidin. Dual-biotinylated λ-DNA was flowed in to form DNA bridges, and the chamber was, subsequently, incubated with QD-labelled TelK monomers (here, 340 nM). QDs stuck to the glass surface were used as reference spots to ensure that drift and background motion were minimal. TelK concentrations used in all TIRF experiments ranged from 70 to 1350 nM. (b) Kymograph of QD-labelled TelK on λ-DNA showing mobile (1–31 s) and stationary (32–50 s) states after analysis and background subtraction with Gaussian fitting of spots. (c) Fluorescence intensity corresponding to the kymograph. The intensity of the mobile TelK doubles as it becomes immobile along the λ-DNA bridge. QD-blinking events (arrows), known to occur for single QDs, are observed only before TelK immobilization. (d) Diffusion coefficient and lifetime for blinking fluorescence spots (n = 114, red) and non-blinking spots (n = 81, blue). The shaded area represents the limit of sensitivity of our assay. The distribution of diffusion coefficients (right panel) is bimodal, with blinkers diffusing approximately four orders of magnitude faster than non-blinkers. As shown in the lifetime distributions (bottom panel), blinking spots also remained DNA-bound for shorter times (4.2 s) than non-blinkers (>50 s).