FIGURE 2.
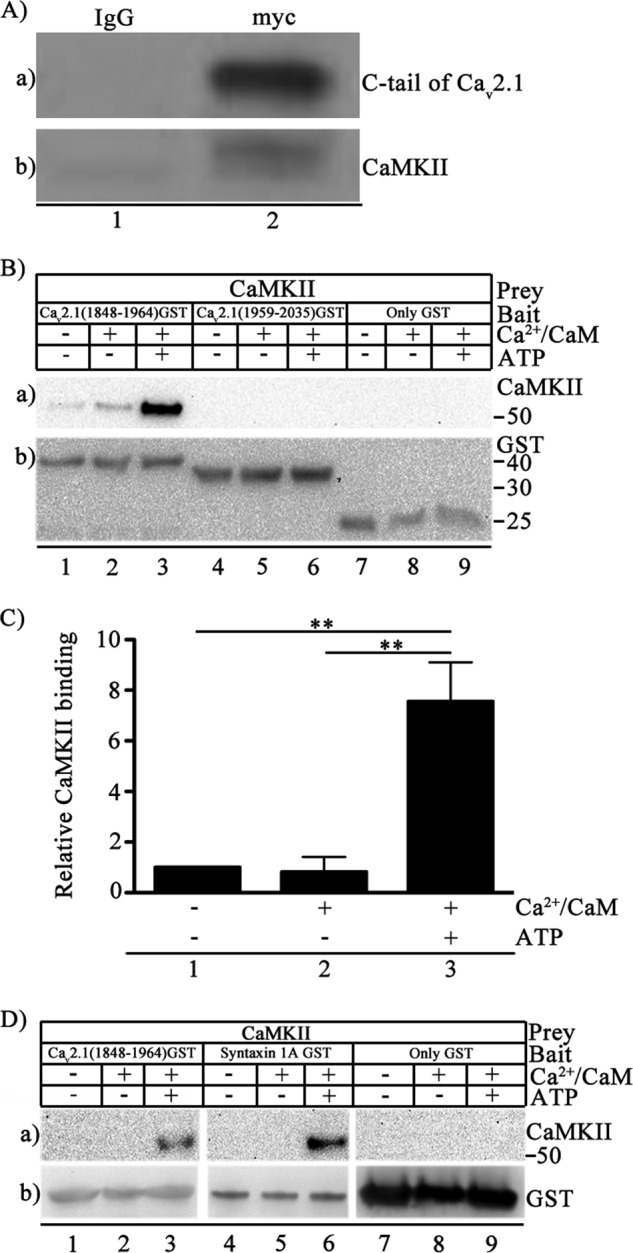
Binding of CaMKII to CaV2.1 channels. A, Myc-tagged Cav2.1(1764–2211) was expressed in tsA-201 cells, and immunoprecipitation was carried out with control IgG (lane 1) or anti-myc antibody (lane 2). a, immunoblotting with anti-myc. b, immunoblotting with anti-CaMKII antibody. B, binding of autophosphorylated CaMKII to Cav2.1 channel. Left to right, CaV2.1(1848–1964), CaV2.1(1959–2035), or GST alone were incubated with Mg2+ (5 mm) and CaMKII (20 nm) in the presence/absence of Ca2+/CaM (5 μm) and ATP (1 mm) as indicated. a, binding of CaMKII was probed using anti-CaMKII. b, the same blot after stripping and re-probing using anti-GST antibody to show equal loading of CaV2.1(1848–1964), CaV2.1(1959–2035), or GST alone. C, quantitation of relative CaMKII binding to CaV2.1(1848–1964) using anti-CaMKII under the indicated conditions (means ± S.E.; **, p < 0.01 by Student's t test; n = 4). D, left to right, CaV2.1(1848–1964) (lanes 1–3), syntaxin 1A (lanes 4–6), or GST (lanes 7–9) were incubated with CaMKII (20 nm) and Mg2+ (5 mm) in the presence/absence of Ca2+/CaM (5 μm) and ATP (1 mm) as indicated. a, binding of CaMKII probed using anti-CaMKII. b, the same blot after stripping and re-probing using anti-GST to show loading of CaV2.1(1848–1964), syntaxin 1A, or GST alone. The section of each blot containing GST-labeled proteins or GST itself was aligned for presentation even though the molecular weights are different.
