FIGURE 8.
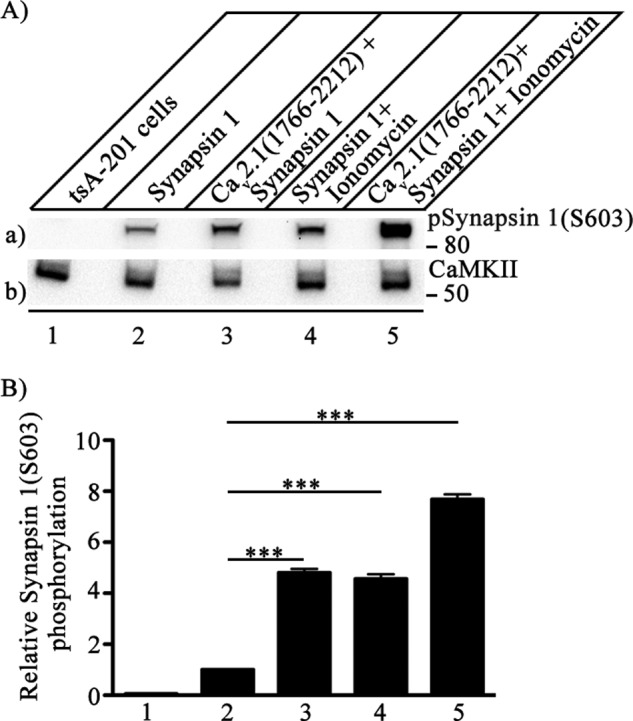
Phosphorylation of synapsin-1 by CaMKII bound to the C-terminal domain of Cav2.1 channels. CaV2.1(1766–2122) was co-expressed in tsA-201 cells along with synapsin-1, and phosphorylation of synapsin-1 was measured with anti-phosphosynapsin-1(Ser-603). A, left to right, untreated control tsA-201 cells (lane 1), cells single-transfected with synapsin-1 (lane 2), cells double-transfected with C-terminal domain of Cav2.1 (1766–2212) channel and synapsin-1 (lane 3), cells single-transfected with synapsin-1 and treated with 5 μm ionomycin to allow Ca2+ entry for 15 min before lysis (lane 4), and cells double-transfected with C-terminal domain of Cav2.1 (1766–2212) channel and synapsin-1 additionally treated with 5 μm ionomycin to allow Ca2+ entry for 15 min before lysis (lane 5). a, phosphorylation of synapsin-1 was assayed by immunoblotting with anti-phosphosynapsin-1(Ser-603) antibody. b, the same blot after stripping and re-probing with anti-CaMKII antibody to show equal loading of CaMKII. B, quantitation of relative synapsin-1(Ser-603) phosphorylation under the indicated conditions (mean ± S.E.; ***, p < 0.001 by Student's t test; n = 5).
