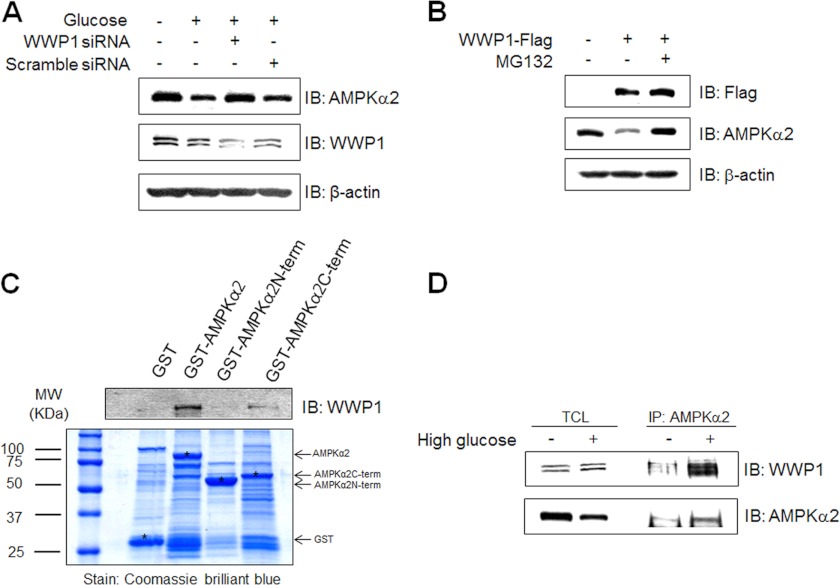
FIGURE 4.
Expression level of AMPKα2 is regulated by expression status of WWP1. A, C2C12 cells were transiently transfected with 50 nm siRNA WWP1 and scramble siRNA for 48 h and then exposed to 30 mm glucose incubation for 48 h. The cell lysates (20 μg) were analyzed via Western blotting for anti-AMPKα2, anti-WWP1 antibodies. The anti-β-actin antibody was a protein loading control. B, C2C12 cells were pre-treated 5 μm MG132 for 1 h and then cells were transiently transfected with Flag-WWP1 for 48 h. The cell lysates (20 μg) were analyzed via Western blotting for anti-AMPKα2, anti-Flag antibodies. The anti-β-actin antibody was a protein loading control. C, C2C12 cells were prepared and incubated with GST fusion proteins of various AMPKα2. Formed protein complexes were isolated by glutathione beads, washed three times with washing buffer, and analyzed by SDS/PAGE and subsequent Coomassie staining. The pull-down sample was also immunoblotted with anti-WWP1 antibody. D, C2C12 cells were exposed to 30 mm glucose incubation for 48 h. The cell lysates (1 mg) of normal and high glucose conditions C2C12 cells were immunoprecipitated with anti-AMPKα2 antibody. The immunoprecipitates were analyzed via Western blotting for anti-WWP1 and AMPKα2 antibodies.