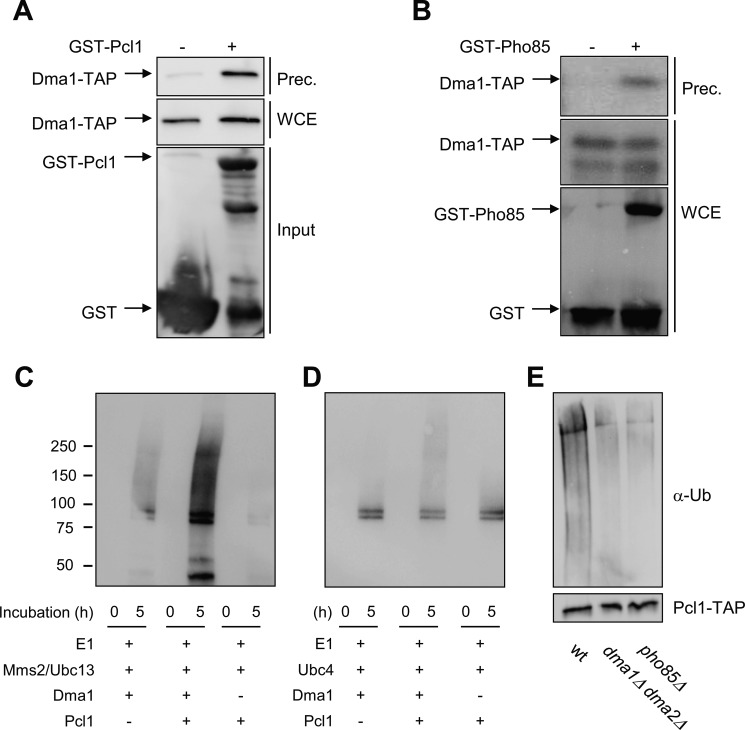
FIGURE 3.
Dma1 interacts and ubiquitinates Pcl1. A, binding in vitro assay. Recombinant GST-Pcl1 was purified from E. coli and incubated with yeast cell extracts of the strain YPC708 (with a genomic TAP-tagged Dma1) for 45 min, and purified with glutathione-Sepharose beads. Samples were analyzed by immunoblot analysis to detect Dma1-TAP proteins. WCE, whole cell extract; Input, quantity of Pcl1 used in the trapping. B, Pcl1-Pho85 complexes co-immunoprecipitate with Dma1 in vivo. Yeast extracts from strains containing untagged PHO85 or GST-PHO85 and TAP-tagged DMA1 (from the chromosomal locus) were precipitated with glutathione-Sepharose beads, and then probed using specific antibodies. C and D, Pcl1 is ubiquitinated in vitro by Dma1. Ubiquitination assays were done by in vitro reconstitution of E1-E2-Dma1 complexes (see “Experimental Procedures”). Panel C shows Dma1 activity associated to the Mms2-Ubc13 E2 complexes. Panel D shows Dma1 activity associated to the Ubc4 E2 enzyme. The reaction was started at time 0 by adding ATP. Samples were taken at 0 and 5 h and analyzed for ubiquitination levels by immunoblotting using α-Ub antibody. E, Pcl1 is ubiquitinated in vivo in a Dma1-dependent manner. The indicated strains carrying a centromeric plasmid with PCL1-His6x-TAP expressed under the GAL promoter was grown for 4 h in synthetic complete medium with galactose as a carbon source. The same amount of immunopurified Pcl1-His was separated by SDS-PAGE gels and visualized by immunoblot using an ubiquitin antibody (top panels). To validate the levels of Pcl1, the blot was also analyzed using an α-TAP antibody (bottom panel).