FIGURE 4.
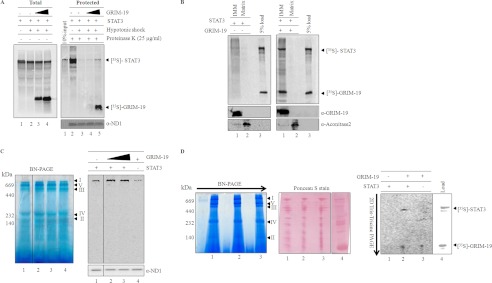
GRIM-19-mediated association of STAT3 with complex I. A, labeled STAT3 was imported into mitochondria for 60 min in the absence (left panel lanes 1 and 2; right panel, lanes 2 and 3) or presence of GRIM-19 (left panel, lanes 3 and 4; right panel, lanes 4 and 5), and mitoplasts were generated by hypotonic treatment of mitochondria. Mitochondria (left panel, lane 1; right panel, lane 2) and the resulting mitoplasts (left panel, lanes 2–4; right panel, lanes 3–5) were either left untreated (left panel) or treated with proteinase K (right panel). After inhibiting the protease, mitochondria and mitoplasts fractions were re-isolated and separated on SDS-PAGE and analyzed by phosphor imager. As a loading control, the right panel fractions were probed with antibodies specific for ND1. B, to demonstrate the association of co-imported STAT3 (with GRIM-19) with IMM, mitoplasts were further separated into IMM and matrix. The equivalent fractions of IMM and matrix were separated on SDS-PAGE and analyzed by phosphorimaging. Left and right panels represent the IMM associated STAT3 in the absence and presence of GRIM-19 respectively. To confirm the separation of IMM and matrix fractions, these fractions were also probed with inner membrane-specific antibody, GRIM-19, and matrix-specific antibody, aconitase (bottom panels). C, to analyze complex I association of STAT3, import of radiolabeled STAT3 was performed without (lane 1) or with increasing concentrations of unlabeled GRIM-19 (lanes 2 and 3). Import of labeled GRIM-19 (lane 4) was used as a positive control. After import, mitochondria were re-isolated and solubilized in 1% dodecyl maltoside buffer, and electron transport chain components were separated by BN-PAGE. Association of STAT3 with the components of the electron transport chain was detected by using phosphorimaging (C, right panel). Immunoblot, with ND1-specific antibody, was performed to show the equal loading of protein in all the fractions. D, to provide direct evidence for STAT3 association with complex I, in vitro import of labeled STAT3 (lane 1) or STAT3/GRIM-19 (lane 2) or GRIM-19 (lane 3) was performed, and these imported samples were protease-treated (25 μg/ml). After inhibiting the protease, the mitochondria samples were solubilized in 1% dodecyl buffer and separated on BN-PAGE (left panel) in the first dimension. For the second dimension, complex I bands were excised and separated on Tris-Tricine PAGE (right panel) and analyzed by phosphorimaging. The Ponceau S stained blot shows equal amount of protein in all lanes (middle panel). Lane 4 represents the in vitro translated 35S-labeled products of STAT3 and GRIM-19 to identify the STAT3 and GRIM-19 on two-dimensional-PAGE.
