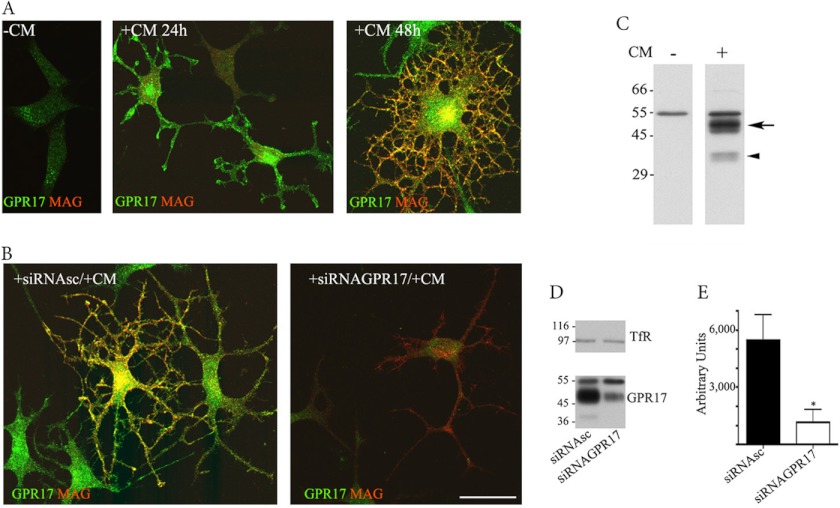
FIGURE 1.
GPR17 expression in Oli-neu cells. Oli-neu cells were cultured in Sato medium ([minus]CM) or conditioned medium (+CM, A) or transfected with scrambled siRNA (+siRNAsc) or a specific siRNA targeted against GPR17 (+siRNAGPR17) and then incubated in CM (+CM, B). At selected time points, the cells were fixed and double-immunolabeled with the anti-Ct-GPR17 antibody (∼1 μg/100 μl; green) and a monoclonal antibody against MAG (1:400, red) followed by fluorochrome-conjugated secondary antibodies. Images were recorded using a confocal microscope; merged images selected from four independent experiments are shown. Note the morphological changes and the increase in GPR17-labeling in Oli-neu cells maintained in medium supplemented with CM. Immunoreactivity for GPR17 was largely reduced upon transfection with a specific siRNA. Scale bar = 20 μm. In C, total membrane extracts (30 μg proteins) of Oli-neu cells cultured in Sato medium or CM (indicated as CM − or +) were analyzed by Western blotting using the anti-Ct-GPR17 antibody (1 μg/ml); the arrow and arrowhead indicate bands of 46–48 and 38 kDa, respectively, specifically immunodetected in differentiated cells. In D, total membrane extracts (30 μg) of Oli-neu cells cultured for 48 h in CM with scrambled siRNA (siRNAsc) or siRNAGPR17 were immunoblotted using antibodies against TfR (1:1000 dilution) or GPR17. Note the specific reduction of the 38 and 46–48 kDa bands after GPR17 knockdown, whereas Tfr and the 57-kDa band are unchanged. E, quantitative analysis of the intensity of the 46–48-kDa bands detected in Western blots of control and silenced cells; the data are expressed as arbitrary units and represent the mean values ±S.E. of three independent experiments (*, p < 0.05).