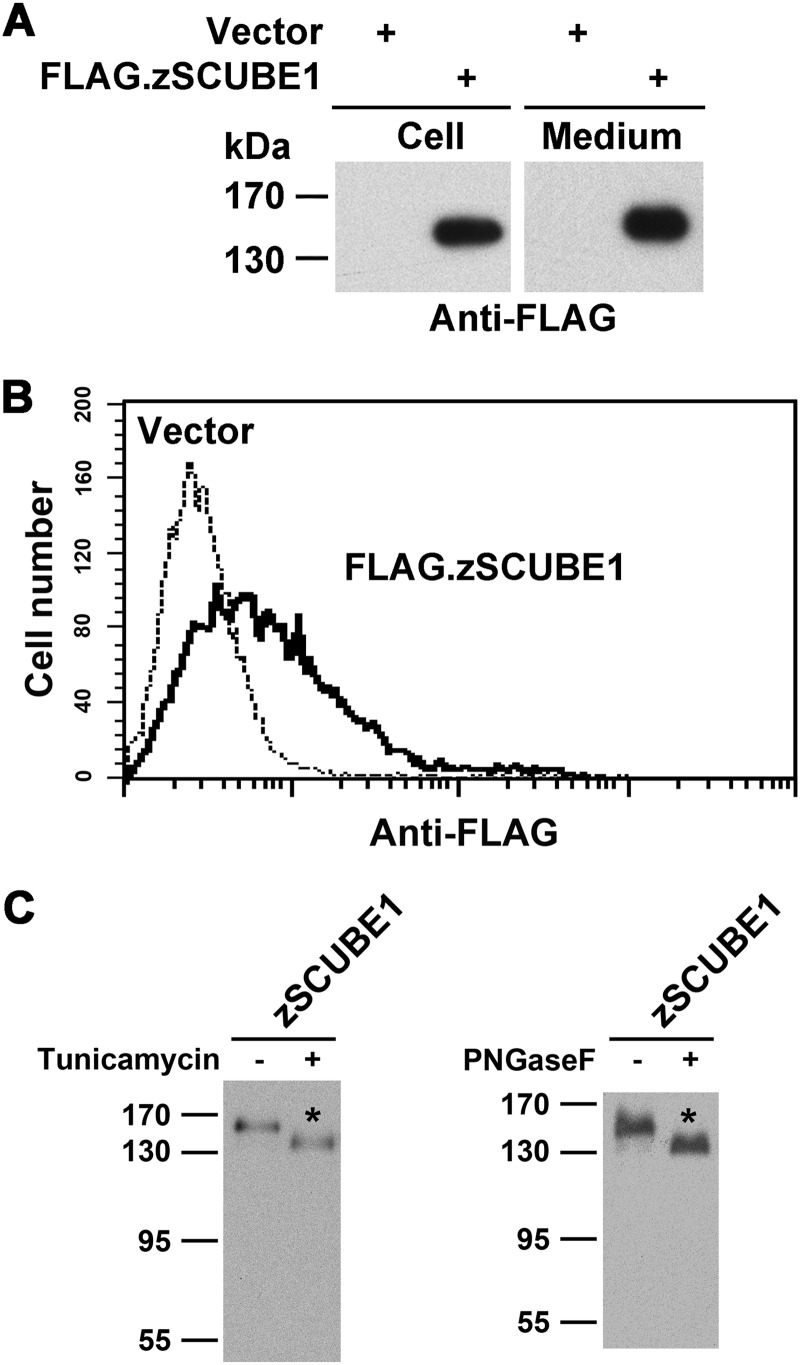
FIGURE 2.
Overexpressed zebrafish Scube1 is a secreted, surface-anchored glycoprotein in HEK-293T cells. A, secretion of zebrafish Scube1 into the conditioned medium. HEK-293T cells were transfected with empty vector (Vector) or expression plasmid encoding FLAG-tagged zebrafish Scube1 (FLAG.zSCUBE1). Forty-eight hours post-transfection, the serum-free conditioned medium was collected, and cells were detached with phosphate-buffered saline/EDTA. Samples from cell lysate (Cell) or the serum-free conditioned culture medium (Medium) were separated by SDS-PAGE and transferred to polyvinylidene difluoride membranes. Recombinant FLAG.zSCUBE1 protein was detected by Western blot analysis with anti-FLAG antibody. B, cell surface expression of zebrafish Scube1 protein. HEK-293T cells were transfected with empty vector (Vector) or expression plasmid encoding FLAG.zSCUBE1 protein. Forty-eight hours post-transfection, cells were detached and probed with anti-FLAG antibody and subjected to flow cytometry analysis. C, N-linked glycosylation of zebrafish SCUBE1. HEK-293T cells were transfected with expression vector encoding FLAG.zSCUBE1 protein. Transfected cells were cultured in the absence (−) or presence (+) of tunicamycin (an inhibitor of N-glycosylation) for 24 h (left). Alternatively, cell lysates were left untreated (−) or treated (+) with peptide N-glycosidase F (PNGaseF) to remove the N-linked oligosaccharide chain (right). Cell lysates derived from each treatment were examined by Western blot analysis with anti-FLAG antibody.