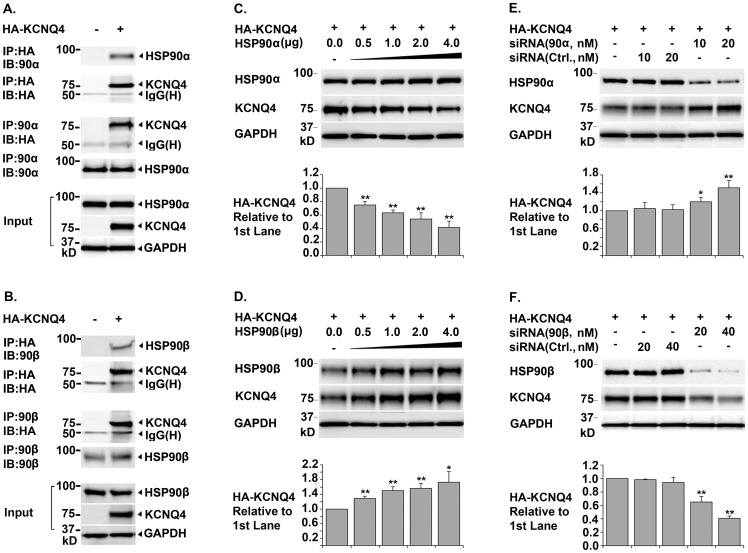
Figure 1. Opposite Roles of Molecular Chaperones HSP90α and HSP90β in KCNQ4 Biogenesis.
A. & B. Co-immunoprecipitation of KCNQ4 channels with the molecular chaperones HSP90 & HSP90β HEK293T cells were transfected with 0.4 µg of pCMV6-XL5-HA-KCNQ4 (+) or pCMV6-XL5 alone (-) and harvested 24 hrs post transfection. Immunoprecipitation (IP) was carried out respectively using a mouse monoclonal antibody against the HA tag in the first extracellular loop in KCNQ4 channels or an antibody against HSP90α (or HSP90β). Precipitated proteins were then analyzed by Western blot (IB) using the indicated antibodies; cell lysates were tested in parallel as input controls. Both HSP90α and HSP90β were co-immunoprecipitation with the KCNQ4 channels and vice versa, indicating that these two chaperones are associated with KCNQ4 channels. C. & D. Effects of over-expression of HSP90α or HSP90β on total KCNQ4 level HEK293T cells were transfected with 0.4 µg of pCMV6-XL5-HA-KCNQ4 (+) and various amount of HSP90α or HSP90β cDNA as indicated. The cells were then lysed in NP40 lysis buffer 24 hrs post transfection and the total amount of KCNQ4 proteins in the cell lysates were assessed by Western blot. Over-expression of HSP90β resulted in a significant increase, while over-expression of HSP90α led to a marked decrease in total KCNQ4 level. E. & F. Effects of siRNA knockdown of HSP90α or HSP90β on total KCNQ4 level HEK293T cells were transfected with 0.4 µg of pCMV6-XL5-HA-KCNQ4 cDNA (+) and various amount of siRNA specifically targeting HSP90α or HSP90β as indicated. Non-specific siRNA (Ctrl.) were used in parallel as negative controls. Total KCNQ4 proteins were assessed by Western blot 48 hrs post transfection. Specific knockdown of HSP90β led to a dramatic decrease, whereas knockdown of HSP90α resulted in a marked increase in total KCNQ4 level. Each data point in the bar graphs represents the mean ±SD of three experiments (* p≤0.05, ** p≤0.01).