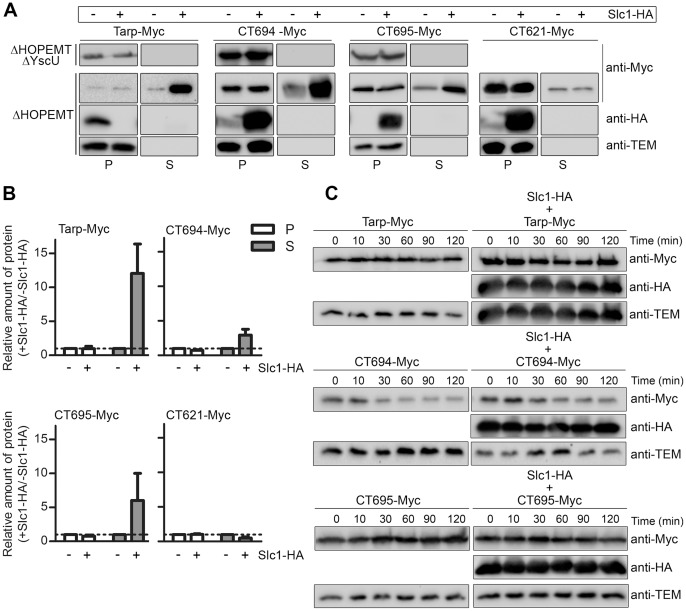
Figure 3. Slc1 promotes type III secretion (T3S) of Tarp, CT694, and CT695 in Y. enterocolitica.
(A) T3S-deficient Y. enterocolitica ΔHOPEMT ΔYscU and T3S-proficient ΔHOPEMT strains expressing the indicated proteins were incubated in T3S-inducing conditions [42]. Proteins in culture supernatants (S - secreted proteins), and in bacterial pellets (P - non-secreted proteins) from ∼5×108 and ∼5×107 bacteria, respectively, were analyzed by immunoblotting with the indicated antibodies. Immunodetection of TEM-1 β-lactamase (encoded by pBAD/Myc-His–derived plasmids expressing Myc-tagged proteins) ensured that presence of proteins in culture supernatants was not a result of bacterial lysis or contamination. (B) The amount of protein in bacterial pellets and in secreted fractions of assays done with ΔHOPEMT-derived strains was estimated by densitometry analyses of immunoblot images. We calculated the ratio between the amounts of each protein (Tarp-Myc, CT694-Myc, CT695-Myc or CT621-Myc) in the presence of Slc1-HA relative to when the proteins were expressed alone (+ Slc1-HA/− Slc1-HA). The dashed line indicates a ratio of 1. Data are the mean ± SEM from 3 independent experiments. (C) Y. enterocolitica ΔHOPEMT strains expressing the indicated proteins were grown in non-secreting conditions (see Materials and Methods). Chloramphenicol was added (time = 0 min) to stop bacterial protein synthesis. Samples were then taken at the depicted time points and analyzed by immunoblotting with the indicated antibodies.