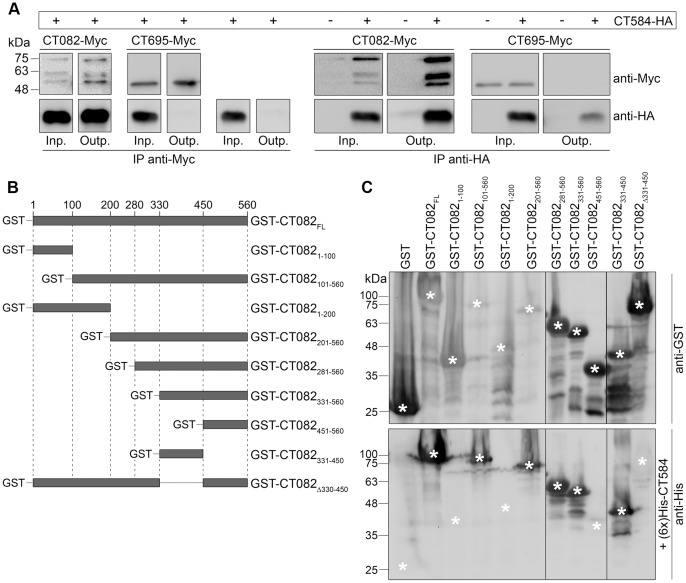
Figure 5. CT584 interacts with a central region of CT082.
(A) Y. enterocolitica ΔHOPEMT strains expressing the indicated proteins were grown in non-secreting conditions. The bacterial cells were lysed and proteins in the lysate supernatants (input) were immunoprecipitated with mouse monoclonal anti-Myc or anti-HA antibodies (as depicted) bound to Protein G agarose beads (output). The input (Inp.) and output (Outp.) fractions from the immunoprecipitations (IPs) were analyzed by immunoblotting with rabbit polyclonal anti-Myc and rat monoclonal anti-HA antibodies. (B) Scheme of the truncated GST-tagged CT082 proteins analyzed to determine the binding region of CT584. (C) Protein overlay binding assays to identify the binding region of CT584 within CT082. Two identical SDS-PAGE were loaded with extracts of E. coli expressing the indicated GST fusion proteins. The electrophoresed proteins were transferred onto nitrocellulose membranes. One membrane was immunodetected with anti-GST antibodies while the other was probed with purified (6x)His-CT584 before being immunodetected with anti-His antibodies. Asterisks indicate the position corresponding to the predicted migration on SDS-PAGE of full-length GST fusion proteins (upper image) and the predicted position where an anti-His-dependent signal should appear if (6x)His-CT584 would bind the GST fusion proteins (lower image).