Abstract
GABAA receptors are critically involved in hippocampal oscillations. GABAA receptor α1 and α2 subunits are differentially expressed throughout the hippocampal circuitry and thereby may have distinct contributions to oscillations. It is unknown which GABAA receptor α subunit controls hippocampal oscillations and where these receptors are expressed. To address these questions we used transgenic mice expressing GABAA receptor α1 and/or α2 subunits with point mutations (H101R) that render these receptors insensitive to allosteric modulation at the benzodiazepine binding site, and tested how increased or decreased function of α subunits affects hippocampal oscillations. Positive allosteric modulation by zolpidem prolonged decay kinetics of hippocampal GABAergic synaptic transmission and reduced the frequency of cholinergically induced oscillations. Allosteric modulation of GABAergic receptors in CA3 altered oscillation frequency in CA1, while modulation of GABA receptors in CA1 did not affect oscillations. In mice having a point mutation (H101R) at the GABAA receptor α2 subunit, zolpidem effects on cholinergically induced oscillations were strongly reduced compared to wild-type animals, while zolpidem modulation was still present in mice with the H101R mutation at the α1 subunit. Furthermore, genetic knockout of α2 subunits strongly reduced oscillations, whereas knockout of α1 subunits had no effect. Allosteric modulation of GABAergic receptors was strongly reduced in unitary connections between fast spiking interneurons and pyramidal neurons in CA3 of α2H101R mice, but not of α1H101R mice, suggesting that fast spiking interneuron to pyramidal neuron synapses in CA3 contain α2 subunits. These findings suggest that α2-containing GABAA receptors expressed in the CA3 region provide the inhibition that controls hippocampal rhythm during cholinergically induced oscillations.
Key points
Hippocampal oscillations are thought to be important for memory encoding and retrieval and depend on inhibition via GABA synapses.
GABAA receptor subunits are differentially expressed throughout the hippocampal circuitry. Here we address which subunit controls cholinergically induced fast network oscillations and where it is expressed.
By selectively increasing and decreasing the function of α1 and α2 subunits, we find that hippocampal oscillations are controlled by α2 subunits expressed in CA3.
Synapses from fast spiking interneurons to pyramidal cells in CA3 that provide the perisomatic inhibition necessary for fast network oscillations contain GABAA receptors with the α2 subunit.
Our data suggest that α2-containing GABA receptors in CA3 have an important role in rhythmic hippocampal activity and thereby possibly in cognitive processing.
Introduction
Gamma-band oscillations (∼30–100 Hz) in the hippocampus are thought to be important for memory encoding and retrieval (Lisman & Idiart, 1995; Montgomery & Buzsaki, 2007) and are usually observed on top of τ oscillations (Bragin et al. 1995; Lisman & Idiart, 1995; Csicsvari et al. 2003; Cardin et al. 2009). Two different gamma generators have been identified for the hippocampus in vivo, i.e. from the entorhinal cortex and within the CA3–CA1 region (Bragin et al. 1995; Csicsvari et al. 2003). In vitro studies showed that the local generator of gamma oscillations within the CA3 region can be activated by muscarinic acetylcholine receptor activation (Fisahn et al. 1998; Mann et al. 2005). These oscillations depend on both AMPA and GABAA receptors (GABAA-R) in the perisomatic region of CA3 pyramidal neurons (Mann et al. 2005) and several pyramidal neuron-targeting interneuron types fire in phase with oscillations (Klausberger et al. 2003; Hajos et al. 2004). The shape of oscillations in the local field potential (LFP) is mainly determined by large phase-coupled synaptic inhibitory currents on CA3 pyramidal neurons (Oren et al. 2006, 2010), whereby cycle-to-cycle variations in inhibition determine the length of a single wave (Atallah & Scanziani, 2009). Inhibitory GABAergic synapse kinetics and subcellular location of GABAA-Rs depends mainly on the α subunits of this receptor (Banks & Pearce, 2000; Hutcheon et al. 2000; Nyiri et al. 2001; Vicini et al. 2001; Klausberger et al. 2002; Bosman et al. 2005). In the CA1 area, depending on type of presynaptic interneuron, GABAergic synapses on pyramidal neurons contain either mainly α1 or α2 subunits (Nyiri et al. 2001; Klausberger et al. 2002), while this is not clear for GABAergic synapses on CA3 pyramidal neurons. At present, it is unknown which GABAA-R α subunit is controlling the power and rhythm of cholinergically induced oscillations. Furthermore, it is not known whether these oscillations in CA1 depend on local feedback inhibition or whether GABAA-Rs on CA3 pyramidal neurons determine the shape of the LFP in CA1.
To address this issue, we made use of GABAA-R subunit knockout mice and transgenic mice in which either α1 subunits, α2 subunits or both were insensitive to allosteric modulation at the benzodiazepine binding site (H101R mutation) (McKernan et al. 2000; Dias et al. 2005), and tested how the kinetics of GABAA-R with either an α1 or an α2 subunit affect hippocampal oscillations. In α1 knockout mice handling-induced tremors are observed (Sur et al. 2001), while no behavioural abnormalities have been reported for mutant mouse lines used in this study. We find that inhibitory postsynaptic currents (IPSCs) in pyramidal neurons in CA3 are mediated mainly by GABAA-Rs containing α2 subunits and that kinetics of these receptor types, in contrast to kinetics of α1 containing receptors, control cholinergically induced oscillations in the hippocampus network. The α2 subunit is present at synapses of perisomatic targeting fast spiking interneurons thought to provide the inhibitory input required for fast network oscillations.
Methods
Ethical approval
All experimental methods involving animals were approved by the animal welfare committee of our university (DierExperimentenCommissie), and in accordance with Dutch and European law. The experiments comply with the policies and regulations as required by The Journal of Physiology (Drummond, 2009)
Slice preparation
All mutant mice and their wild-type littermates were from an original mixed genetic background of C57BL6 and 129SvEv and were back-crossed to a C57BLl6 background (five to 10 generations). Techniques for creation of mutant lines have been previously described: α1 knockout (Sur et al. 2001), α2 knockout (Boehm et al. 2004), α1H101R (McKernan et al. 2000) and α2H101R (Dias et al. 2005). Mice carrying the H101R point mutation in both α1 and α2 were obtained by crossing double heterozygous mice born from homozygous α1H101R and α2H101R intercrosses. Mutant mice and wild-type littermates were identified using PCR analysis. Wild-type littermates were used for comparison with transgenic mice; all other experiments were performed on C57Bl6 mice. After decapitation without anaesthesia, brains were quickly removed and stored in ice-cold artificial cerebrospinal fluid (ACSF) containing (mm): 126 NaCl, 3 KCl, 10 glucose, 26 NaHCO3, 1.2 NaH2PO4, 1 CaCl2 and 3 MgSO4 (carboxygenated with 5% CO2/95% O2) or a modified slicing solution with low sodium concentrations used for paired recordings and recordings in adult animals containing (mm): 110 choline chloride, 11.6 sodium ascorbate, 7 MgCl2, 3.1 sodium pyruvate, 2.5 KCl, 1.25 NaH2PO4, 0.5 mm CaCl2, 26 NaHCO3 and 10 glucose (carboxygenated with 5% CO2/95% O2). Horizontal hippocampal slices (400 μm) from young (P13–18) or adult (P55–70) animals were cut in ice-cold ACSF using a microtome (Microm, Waldorf, Germany). After preparation, slices were stored for at least 1 h in ACSF containing 2 mm CaCl2 and 2 mm MgSO4. Next the slices were mounted on planar multi-electrode arrays with 150 μm spacing (MED-P5155, Alpha MED Sciences, Osaka, Japan) or 200 μm (200/30-T-gr and pMEA200/30iR, Multichannel Systems, Reutlingen, Germany) with a polyethylene coating (Sigma-Aldrich, St Louis, MO, USA). Slices were left to attach properly to electrodes for at least 1 h in a chamber with humidified carbogen gas before they were placed in the recording unit. For recording oscillations in adult slices we used perforated arrays with a 200 μm spacing (pMEA200/30iR) and slices were attached on the electrode array by 30 mbar under pressure.
Electrophysiology
LFPs were measured in four slices simultaneous with four recording units at the same time using the multichannel system or in combination with patch clamp recordings in the MED64 system.
During recording, slices were perfused with ACSF at a flow rate between 4 and 5 ml min−1 and were kept at 30°C and in submerged conditions; for adult slices, the flow rate underneath the slice was 1 ml min−1. ACSF containing 25 μm carbamoylcholine chloride (Carbachol; Sigma-Aldrich) was perfused for at least 45 min or added after the mounting of the slices on to the multi-electrode grids. Spontaneous field potentials from all 64 recording electrodes were acquired simultaneously at 20 kHz (Alpha MED Sciences) or with 60 electrodes at 1 kHz (Multichannel Systems, Reutlingen, Germany).
Pyramidal neurons in CA3 and CA1 neurons were recorded in whole cell mode using a Multiclamp 700B amplifier (Molecular Devices, Sunnyvale, CA, USA). Borosilicate glass (Harvard Apparatus, Holliston, MA, USA) electrodes with tip resistances of 2–5 MΩ were filled with intracellular solution containing (mm): 140 potassium gluconate, 1 KCl, 10 Hepes, 4 potassium phosphocreatine, 4 ATP-Mg and 0.4 GTP (pH adjusted to 7.3 with KOH). To ensure temporal alignment between the multi-electrode and single cell electrophysiological signals, the current or voltage signals from the patch clamp amplifiers were recorded on one channel of the MED64 system, using a custom-made interface device. IPSCs during oscillations were recorded at +20 mV holding potential.
Local injections of 1 mm tetrodotoxin citrate (TTX; Abcam, Cambridge, UK) and 1 mm zolpidem (Duchefa, Haarlem, The Netherlands) were done with borosilicate glass electrodes with tip resistances of 1–2 MΩ. Injections were done by giving 25–30 mbar overpressure for 1 min in the stratum pyramidale of either CA3 or CA1. Zolpidem was dissolved in DMSO and TTX dissolved in ACSF.
Unitary connections of fast spiking interneurons to pyramidal neurons
Recordings were made from fast spiking interneurons with their soma in stratum oriens/pyramidale and from pyramidal neurons. Electrodes were filled with an intracellular solution containing (mm): 70 potassium gluconate, 70 KCl, 4 Mg-ATP, 4 phosphocreatine, 0.4 GTP, 0.5 EGTA, 10 Hepes and 0.5% biocytin (pH 7.3, KOH).
Glutematergic events were blocked using DNQX (10 μm). Interneuron activity was recorded in current clamp, while pyramidal cells were voltage clamped at −70 mV. In connected cell pairs a spike in the interneuron resulted in an IPSC in the pyramidal neuron. Owing to the high intracellular chloride concentrations, IPSC were observed as inward current. The presynaptic cell was stimulated with suprathreshold current pulses with an interval of 5 s. After recording slices were transferred to a 4% paraformaldehyde solution. Biocytin was revealed with the chromogen 3,3′-diaminobenzidine tetrahydrochloride using the avidin–biotin–peroxidase method (Horikawa & Armstrong, 1988). Slices were mounted on slides and embedded in mowiol (Clariant GmbH, Frankfurt am Main, Germany). Cells were reconstructed using Neurolucida software (Microbrightfield, Williston, VT, USA), using a ×100 oil objective.
Data analysis
Synaptic events were detected using the Mini Analysis Program (Synaptosoft, Decatur, GA, USA). All other analyses were done using custom-made scripts in Igor Pro (Wavemetrics, Lake Oswego, OR, USA) and Matlab (The Mathworks, Natick, MA, USA). Data were down-sampled to 200 Hz–2 kHz for analysis. Fourier transforms were performed using a 2 s Hanning window. Frequency and power of oscillations were calculated by fitting a Gaussian function to the Fourier transformed data, as noise in the Fourier transform might influence the peak frequency of the oscillations. The average power and frequency of the oscillations were calculated separately for the CA3 region and the CA1 region by taking the average of all electrodes in that region. The power of oscillations is transformed by taking the squared root to obtain a normal distribution. Single cell data were only used for cells that showed significant phase coupling between IPSCs and the LFP of the electrode near the soma of the pyramidal neuron. IPSC τ decay times were calculated by fitting an exponential function to the individual IPSCs, all events with double peaks were rejected from analysis (<10%). All IPSC τ decay times and amplitudes of a single neuron were put in a histogram and fitted with a lognormal function. For statistical analysis the Student's t test or for multiple comparisons an ANOVA with Student–Newman–Keuls post hoc test was used. Data are represented as average ± SEM, n is indicated as number of slices for LFP or number of cells for intracellular recordings. Statistical differences of P < 0.05 are indicated by asterisks in the figures.
Results
Fast network oscillations are reduced in α2−/− mice
Hippocampal fast network oscillations induced by muscarinic receptor activation depend on rhythmic synaptic inhibition of CA3 pyramidal neurons (Fisahn et al. 1998; Mann et al. 2005; Oren et al. 2010). Which GABAA-R subunits are involved in this rhythmic inhibition of these neurons is not known. GABAA-Rs containing α1 or α2 subunits are the most abundant synaptic GABAA-Rs in mouse hippocampus (Laurie et al. 1992; Fritschy & Brunig, 2003; Prenosil et al. 2006). As a first step, to test whether hippocampal fast network oscillations induced by muscarinic receptor stimulation depend on GABAA-Rs containing either α1 or α2 subunits, we studied oscillations in acute hippocampal slices of mice lacking these subunits. The cholinergic agonist carbachol (25 μm) induced fast network oscillations at a frequency of 20.7 ± 0.3 Hz at 30°C. The frequency of these oscillations depends on temperature and at physiological temperatures the frequency falls into the beta/gamma band range (Dickinson et al. 2003; Jansen et al. 2011). In GABAA-R α1 subunit knockout mice (α1−/−) no differences in frequency and power of carbachol-induced oscillations in CA3 were observed compared to wild-type littermates (α1+/+ n= 11 slices, 6 animals, α1−/−n= 15 slices, 6 animals, P= 0.79 and P= 0.43 respectively, unpaired Student's t test; Fig. 1A and C). In addition, the spatial distribution of oscillations in the hippocampal slice was not altered in mice lacking α1 subunits (Fig. 1A, bottom panels). In contrast in mice lacking GABAA-R α2 subunits (α2−/−), the power of oscillations in CA3 was strongly reduced (26.6 ± 6.3 μV Hz−2 in α2+/+, n= 11 slices, 5 animals, 7.0 ± 1.4 μV Hz−2 in α2−/−, n= 10 slices, 5 animals, P < 0.05, unpaired Student's t test; Fig. 1B and D). The frequency of oscillations and the spatial distribution were not affected (Fig. 1B and D). These results suggest that GABAA-Rs containing α2 subunits are involved in cholinergically induced oscillations.
Figure 1. Hippocampal fast network oscillations are strongly reduced in α2−/− mice but not in α1−/− mice.
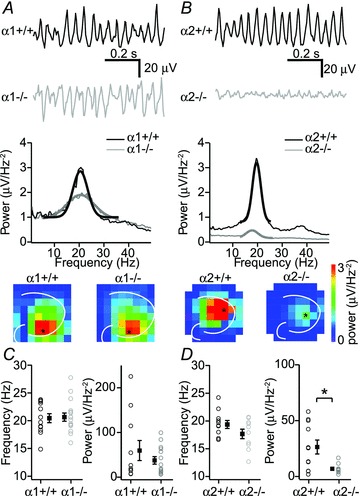
A, example traces of oscillations in CA3 in α1+/+ mice and α1−/− mice (top) and corresponding Fourier transforms (middle) and peak power at different location of the hippocampus (bottom). Traces are from electrodes indicated by an asterix. B, example traces of oscillations in CA3 in α2+/+ mice and α2−/− mice (top) and corresponding Fourier transforms (middle) and peak power at different location of the hippocampus (bottom). C, peak frequency and peak power of oscillations in α1+/+ mice and α1−/− mice (n= 11 for α1+/+ and n= 15 for α1−/−). D, peak frequency and peak power of oscillations in α2+/+ mice and α2−/− mice (n= 15 for α1+/+ and n= 10 for α1−/−). Data show SEM.
Zolpidem modulation of fast network oscillations is largely reduced in α2H101R mice
The α1−/− and α2−/− mice lack these subunits from early development on, which may result in compensatory developmental adaptations that may compromise interpretation of the results. Therefore, to circumvent these potential problems, we made use of transgenic mice that express GABAA-R α1 and/or α2 subunits with point mutations that render these receptors insensitive to allosteric modulation via the benzodiazepine binding site (McKernan et al. 2000; Dias et al. 2005). These mice show normal behaviour and do not suffer from compensatory expression that general GABAA-R knockout mice experience, i.e. function and location of the GABAA-Rs are not affected (Rudolph et al. 1999; McKernan et al. 2000; Prenosil et al. 2006). It is well-established that allosteric modulation of GABAA-Rs, with for instance zolpidem, alters the frequency and power of carbachol-induced fast network oscillations (Fig. 2A and B) (Palhalmi et al. 2004; Cope et al. 2005; Heistek et al. 2010). Zolpidem has a higher affinity for GABAA-Rs containing the α1 subunit: at 100 nm zolpidem modulates α1-containing receptors more than other GABAA-Rs. At 1 μm, zolpidem also modulates α2/α3 containing receptors (Pritchett & Seeburg, 1990). In wild-type mice, zolpidem decreased the frequency of oscillations in CA3 in a concentration-dependent manner (96.1 ± 1.0% and 87.0 ± 1.4%, n= 26 slices, 14 animals with 0.1 and 1 μm zolpidem respectively, Fig. 2A–F). Furthermore, zolpidem increased the power of oscillations within CA3 to 140 ± 15% and 247 ± 32%, n= 26 slices, 14 animals with 0.1 and 1 μm zolpidem respectively (Fig. 2). These effects of zolpidem were similar in CA3 and CA1 region (Fig. 2C, see also Fig. 5). Application of zolpidem did not alter the spatial distribution of oscillations (Fig. 2C). To test the involvement of GABAA-R α1 subunits, zolpidem was applied to hippocampal slices of mice carrying GABAA-R α1 subunits that all had a H101R point mutation (α1H101R), which renders this subunit insensitive to zolpidem. In these hippocampal slices, we still observed a decrease in frequency and an increase in power in CA3 (n= 17 slices 10 animals, P < 0.05, repeated measures ANOVA, Fig. 2D–G). The decrease in frequency was substantially larger in α1H101R mice compared to wild-type mice (92.3 ± 1.9% and 81.0 ± 1.5% of control with 0.1 and 1 μm zolpidem respectively, n= 17 slices 10 animals, P < 0.05, Student–Newman–Keuls; Fig. 2D and F). These data suggest that allosteric modulation of GABAA-Rs through the benzodiazepine binding site on α1 subunits is not involved in the changes in oscillation frequency and power induced by zolpidem.
Figure 2. Changes of frequency and power of fast network oscillations by allosteric modulation of GABAA receptors are absent in α2 H101R mice.
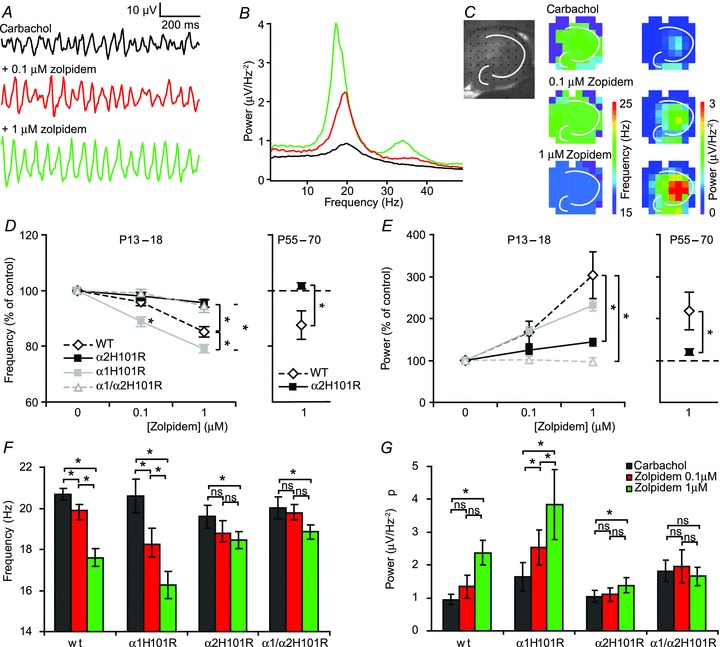
A, example traces of oscillations in CA3 in control and different concentrations of zolpidem in hippocampal slices of wild-type mice at 30°C. B, Fourier transform corresponding to the traces in A. C, left, hippocampal slice placed on a multi-electrode grid. The white lines show the stratum pyramidale and the granular layer of the dentate gyrus. Middle, frequency of fast network oscillations at control and different concentrations of zolpidem in wild-type mice at different electrodes. Right, power of fast network oscillations at control and different concentrations of zolpidem in wild-type mice at different electrodes. D, (left) comparison of zolpidem effects on frequency between different transgenic mice in CA3 in young animals (n= 26, n= 17, n= 24 and n= 18 for wild-type, α1 H101R, α2 H101R and α1 H101R/α2 H101R respectively), (right) zolpidem effects on frequency in adult animals (n= 7 and n= 8 for wild-type and α2 H101R respectively). Data are normalized to control. E, comparison of zolpidem effects on frequency between different transgenic mice in CA3 in young (left) and adult animals (right). Data are normalized to control. F, effects of zolpidem on the frequency of fast network oscillations in different transgenic mice. G, effects of zolpidem on the power of fast network oscillations in different transgenic mice. ANOVA test showed differences between genotypes in both frequency and power (P < 0.05). Data show SEM. WT, wild-type.
Figure 5. Zolpidem effects on decay time kinetics in CA1 pyramidal cells.
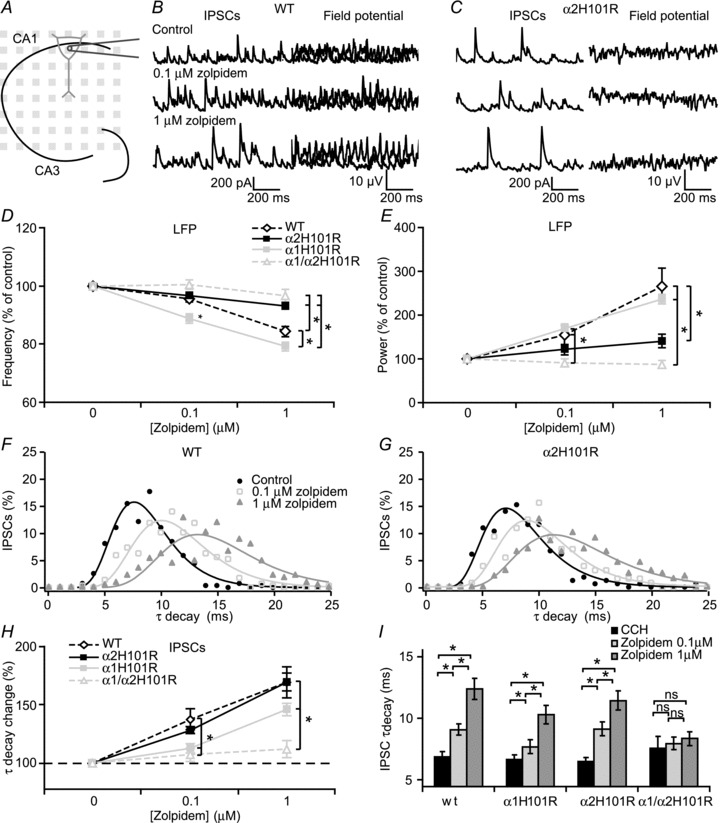
A, experimental setup. B, left, example traces of IPSCs from a CA1 pyramidal cell in control and increasing concentration of zolpidem from a WT mouse. Right, corresponding LFP. C, same as in B, but now in a pyramidal cell from a α2H101R mouse. D, comparison of zolpidem effects on frequency between different transgenic mice in CA1 (n= 25, n= 15, n= 21 and n= 18 for WT, α1H101R, α2H101R and α1H101R/α2H101R, respectively). Data are normalized to control. E, comparison of zolpidem effects on frequency between different transgenic mice in CA1. Data are normalized to control. ANOVA test showed differences between genotypes in both frequency and power (P < 0.05). F, histogram of IPSC decay time kinetics fitted with a lognormal function (line) in a WT mouse. G, histogram of IPSC decay time kinetics fitted with a lognormal function (line) in a WT mouse. H, effects of increasing concentrations of zolpidem on IPSC decay time kinetics in different transgenic mice ANOVA test showed differences between genotypes (P < 0.05, n= 12, n= 11, n= 11 and n= 5 for WT, α1H101R, α2H101R and α1H101R/α2H101R, respectively). Data are normalized to control. I, changes in decay time kinetics by zolpidem. Data show SEM. CCH, carbachol; IPSC, inhibitory postsynaptic current; LFP, local field potential; WT, wild-type.
In contrast, in mice expressing α2 subunits with a H101R point mutation (α2H101R), the effects of allosteric modulation of GABAA-R function by zolpidem in CA3 were strongly reduced compared to wild-type animals (P < 0.05, Student–Newman–Keuls). At 0.1 μm zolpidem there was no effect on frequency or power of oscillations; at 1 μm, zolpidem only a small but significant decrease in frequency (96.7 ± 1.3%, n= 24 slices, 15 animals, P < 0.05) and a moderate increase in power were observed (126.2 ± 9.0%, P < 0.05) (Fig. 2D–G). These results suggest that allosteric modulation of α2 subunit-containing GABAA-Rs mediates the effects of zolpidem on oscillation frequency and power.
In double mutant mice with the H101R point mutations in both the α1 and α2 subunits (α1/α2H101R) results were not significantly different from those in α2H101R mice (P > 0.05, Student–Newman–Keuls), and in these mice 1 μm zolpidem did not increase the power of oscillations (n= 18 slices, 8 animals, P= 0.51, repeated measures ANOVA, Fig. 2D–F). As the expression of α subunits might be developmentally regulated, we also tested the effects of zolpidem in adult mice (P55–70). Similar to the findings in young animals, the effects of 1 μm zolpidem on frequency (101.5 ± 1.2%) and power (121 ± 7%) in adult α2H101R mice were reduced compared to adult wild-type mice (87.7 ± 5.0% and 218 ± 41%, P < 0.05, Student–Newman–Keuls).
Together, these data show that allosteric modulation of GABAA-Rs containing α2 subunits can alter the frequency and power of carbachol-induced oscillations in hippocampus, while the contribution of allosteric modulation of α1 subunit containing receptors to oscillations is marginal.
Location of GABAA receptors involved in modulating hippocampal oscillations
To determine where the α2 subunit-containing GABAA-Rs that affect the frequency and power of oscillations are located, fast network oscillations were manipulated specifically either in CA3 or CA1 region. Local application of 1 mm TTX in the stratum pyramidale of the CA3 region blocked carbachol-induced oscillations within 1 min in the entire hippocampal slice (power decreases to 6 ± 1%, n= 13 slices, 4 animals; Fig. 3A and B; P < 0.05, paired Student's t test). In contrast, local injection of TTX in the stratum pyramidale or in stratum radiatum of the CA1 region did not significantly affect oscillations in either the CA1 or the CA3 region (n= 7 slices, 2 animals; Fig. 3A and B; P= 0.24, paired Student's t test). To make sure that the TTX application in CA1 was effective, we subsequently applied TTX in CA3, which blocked oscillations in all slices. These data indicate that action potential firing of CA1 neurons within the stratum pyramidale may not be the major determinant of local fast network oscillations during cholinergic receptor activation. Instead, synaptic inputs from CA3 neurons may underlie oscillatory field potentials in CA1, which is in agreement with earlier studies showing that the generator of the oscillations is in CA3 (Fisahn et al. 1998; Mann et al. 2005).
Figure 3. Local injection of TTX and zolpidem.
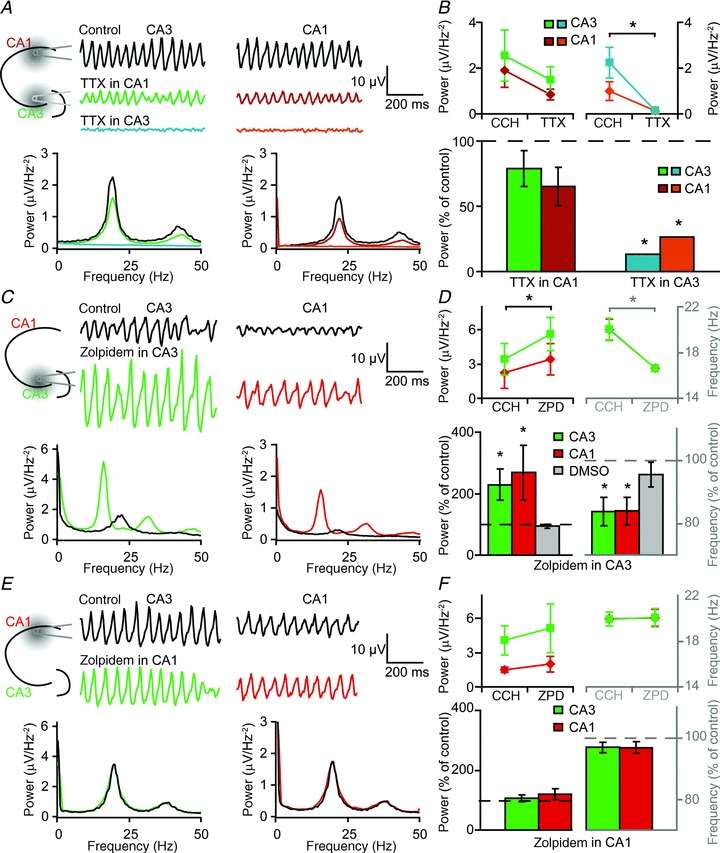
A, schematic view (top left) of experimental setup. TTX (1 mm) was injected with a glass electrode in either CA3 or CA1. Example traces (top right) and corresponding Fourier transforms (bottom) in control and after TTX application in different areas. B, effects of TTX injection in CA3 (n= 14) and CA1 (n= 7) on the power of oscillations in CA3 and CA1 (top), and normalized change in power by TTX (bottom). C, experimental setup (top, left), example traces (top,right) and Fourier transform (bottom) in control and after injection of 1 mm ZPD in CA3. D, effects of ZPD injection in CA3 (n= 5) and on the power and frequency of oscillations in CA3 and CA1 (top), and normalized change in power and frequency by ZPD or DMSO injection in CA3 (bottom). E, experimental setup (top, left), example traces (top,right) and Fourier transform (bottom) in control and after injection of 1 mm ZPD in CA1. F, effects of ZPD injection in CA1 (n= 6) and on the power and frequency of oscillations in CA3 and CA1. Data show SEM. CCH, carbachol; TTX, tetrodotoxin citrate; ZPD, zolpidem.
Next, we tested whether allosteric modulation of GABAA-R function in either the CA3 or CA1 region of the hippocampus affected the frequency of oscillations in these regions. Zolpidem injection (1 mm) in CA3 decreased the frequency (to 83.8 ± 4.5%, P < 0.05 in both CA3 and CA1, paired Student's t test) and increased the power (to 129 ± 51% in CA3 and by 168 ± 89% in CA1, P < 0.05, paired Student's t test) of oscillations in the whole hippocampus (n= 5 slices, 3 animals; Fig. 3C and D), while zolpidem injection in the CA1 region had no effect on the oscillations, neither on the oscillations in CA3 nor in the CA1 region (P= 0.31 and P= 0.28 respectively, paired Student's t test, n= 6 slices, 2 animals, Fig. 3E and F). The solvent DMSO had no effect on frequency and power of oscillations (n= 6 slices, 3 animals, P= 0.37 and P= 0.81, Fig. 3D). These data indicate that allosteric modulation of GABAA-Rs located in the CA3 region affect the frequency of cholinergically induced fast network oscillations in the hippocampus.
Reduced allosteric modulation of inhibitory postsynaptic currents in CA3 pyramidal neurons in α2H101R mice
Earlier work demonstrated that cholinergic fast network oscillations in hippocampus are set by recurrent excitation and perisomatic fast synaptic inhibition of CA3 pyramidal neurons (Mann et al. 2005; Oren et al. 2006). A logical consequence of the findings above is that GABAA-Rs mediating fast synaptic inhibition received by CA3 pyramidal neurons during oscillations should have a strong α2 subunit contribution and allosteric modulation of α2 subunit-containing GABAA-Rs should affect the kinetics of fast synaptic inhibition during oscillations. To test this, we recorded spontaneous GABAergic IPSCs in CA3 pyramidal neurons during carbachol-induced oscillations of different mutant mice and quantified the effects of zolpidem on IPSC kinetics. Both in wild-type and α1H101R mice, the decay time kinetics of IPSCs increased by application of 1 μm zolpidem (to 140 ± 7% (n= 7 cells, 4 animals) and 147 ± 10% (n= 6 cells, 3 animals) respectively; Fig. 4A, B, D–G). In α2H101R and α1/α2H101R mice, the effect of zolpidem on IPSC kinetics was strongly reduced compared to wild-type animals (117 ± 4%, n= 8 cells, 5 animals and 115 ± 6%, n= 7 cells, 3 animals, both P < 0.05, Student–Newman–Keuls, Fig. 4C, D, F and G). These data suggest that α2 subunit-containing GABAA-Rs are strongly involved in fast synaptic inhibition in CA3 pyramidal neurons during oscillations.
Figure 4. Zolpidem effects on decay time kinetics in CA3 pyramidal cells.
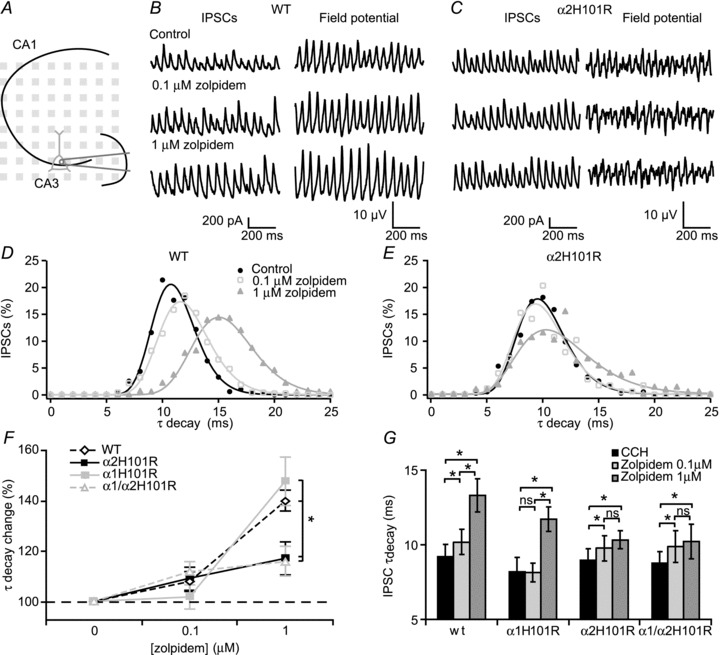
A, experimental setup. B, left, example traces of IPSCs from a CA3 pyramidal cell in control and increasing concentration of zolpidem from a WT mouse. Right, corresponding local field potential. C, same as in B, but now in a pyramidal cell from the α2H101R mouse. D, histogram of IPSC decay time kinetics fitted with a lognormal function (line) in a WT mouse. E, histogram of IPSC decay time kinetics fitted with a lognormal function (line) in a WT mouse. F, effects of increasing concentrations of zolpidem on IPSC decay time kinetics in different transgenic mice. ANOVA test showed differences between genotypes (P < 0.05, n= 7, n= 6, n= 8 and n= 7 for wild-type, α1H101R, α2H101R and α1H101R/α2H101R, respectively). Data are normalized to control. G, changes in decay time kinetics by zolpidem. Data show SEM. CCH, carbachol; IPSC, inhibitory postsynaptic current; WT, wild-type.
Spontaneous IPSCs recorded from CA1 pyramidal neurons during fast network oscillations had faster decay time kinetics on average than CA3 pyramidal neurons (7.2 ± 0.4 ms and 9.2 ± 0.8 ms respectively, P < 0.05, Student's t test; Fig. 5A and B). Although the inhibitory input did not follow every cycle of the oscillations as in CA3 pyramidal cells, the IPSCs were phase locked to the LFP in CA1. Surprisingly, in contrast to IPSCs received by CA3 pyramidal neurons in α2H101R mice, IPSCs recorded in CA1 pyramidal neurons of these mice during fast network oscillations showed substantial allosteric modulation by zolpidem (169 ± 14%, n= 11 cells, 5 animals, P < 0.05, repeated measures ANOVA; Fig. 5C, F–I) and comparable to zolpidem modulation of IPSC kinetics in wild-type animals (169 ± 7%, n= 12 cells, 5 animals, P= 0.98). In the same slices, the modulation of frequency and power of field potential oscillations by zolpidem in the CA1 region of α2H101R mice was strongly reduced compared to wild-type animals (P < 0.05, Student–Newman–Keuls; Fig. 5D and E). These results are in line with findings that α2 subunit-containing GABAA-Rs located in the CA3 region control field potential oscillations in CA1. In CA1 pyramidal neurons of α1H101R mice, allosteric modulation of IPSCs by 100 nm zolpidem was reduced (n= 11 cells, 5 animals, P < 0.05, Student–Newman–Keuls; Fig. 5F–I). At the same time, the effects of zolpidem on the frequency of oscillations in CA1 were larger than in wild-type mice (P < 0.05, Student–Newman–Keuls; Fig. 5A), confirming that IPSC kinetics in CA1 pyramidal neurons are most likely not controlling the properties of oscillations in CA1. In α1/α2H101R double mutant mice, zolpidem effects on decay time kinetics of IPSCs received by CA1 pyramidal neurons were absent (112 ± 8%, n= 5 cells, 3 animals, P≥ 0.26, P < 0.05, repeated measures ANOVA; Fig. 5H and I). As zolpidem did affect IPSC kinetics in α2H101R while the LFP oscillations in CA1 were unaffected, it is unlikely that the α2 subunit-containing GABA receptors contribute substantially to inhibitory currents in CA1 pyramidal neurons during oscillations. In α1H101R mice, modulation of IPSCs by zolpidem was reduced, suggesting that the rhythmic inhibitory inputs on CA1 pyramidal neurons during cholinergic fast network oscillations are predominantly mediated by GABAA-Rs containing α1 receptors.
Unitary GABAergic synapses between fast spiking interneurons and pyramidal neurons in CA3 contain α2 subunits
Several studies indicated that parvalbumin expressing fast spiking interneurons play a critical role in the generation of gamma oscillations (Fuchs et al. 2007; Cardin et al. 2009; Gulyas et al. 2010), while synapses from cholecystokinin expressing interneurons are silenced by carbachol (Gulyas et al. 2010). Electron microscopy studies in CA1 showed that synapses in pyramidal cells from axo-axonic cells and cholecystokinin basket cells contain mainly GABA-Rs with α2 subunits, while fast spiking basket cells contain α1 subunits (Nyiri et al. 2001; Klausberger et al. 2002 but see Kasugai et al. 2010). If the expression of α subunits is the same in the CA3 region, our data seem to oppose the idea that fast spiking interneurons provide inhibition during oscillations. Therefore, we tested whether the synapses between perisomatic targeting fast spiking interneurons and pyramidal neurons in CA3 contain the α2 subunit by making recordings of connected pairs of these two cell types in wild-type, α1H101R and α2H101R mice.
Fast spiking interneurons had their soma in stratum pyramidale or stratum oriens and were identified based on their spike pattern after depolarizing current injections (Fig. 6D). Biocytin reconstruction of these interneurons showed that their axon projected either to stratum oriens/pyramidale or to stratum oriens/radiatum (Fig. 6A). Application of zolpidem increased the decay kinetics of IPSCs induced by a single spike in the fast spiking interneuron to 154 ± 7% (n= 9 cells, 7 animals, Fig. 6B, C and E). In contrast, in α2H101R mice the increase in decay time constant by zolpidem (122 ± 10%, n= 8 cells, 4 animals) was reduced compared to both wild-type animals and α1H101R mice (α1H101R 168 ± 6%n= 3 cells, 2 animals, P < 0.05 ANOVA, with Student–Newman–Keuls post hoc test; Fig. 6B, C and E). Given the important role of perisomatic targeting fast spiking interneurons in the generation of fast network oscillations (Mann et al. 2005; Oren et al. 2006; Fuchs et al. 2007; Cardin et al. 2009; Gulyas et al. 2010), our data may suggest that GABAA-Rs containing α2 subunits in fast spiking interneurons to pyramidal neuron synapses in the CA3 region mediate the fast synaptic inhibition that controls the frequency of hippocampal fast network oscillations.
Figure 6. Synapses between perisomatic targeting fast-spiking interneurons and CA3 pyramidal neurons contain α2 subunits.
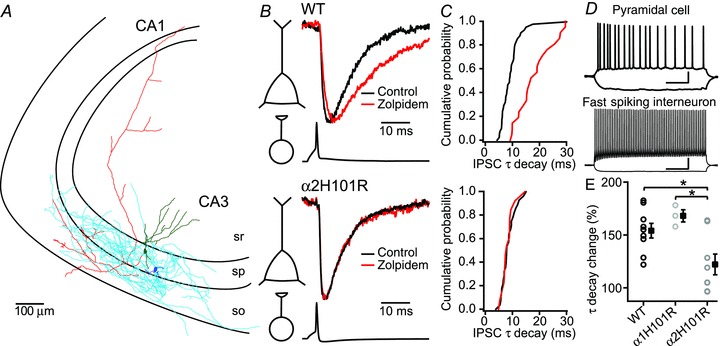
A, reconstruction of a biocytin-filled connected CA3 pyramidal cell and perisomatic targeting fast spiking interneuron (interneuron axon light blue; interneuron soma and dendrites dark blue; pyramidal cell axon red; pyramidal cell dendrites and soma). B, peak scaled average IPSC in a pyramidal cell evoked by a single action potential in an interneuron before and after application of zolpidem in WT (top) and α2H101R mice (bottom). C, cumulative distribution of τ decay times of the cell pair shown in B. D, characteristic spike pattern of pyramidal cell and fast spiking interneuron. Scale bar 200 ms, 20 mV. E, average change in τ decay time by zolpidem (n= 9, n= 3 and n= 8 for WT, α1H101R and α2H101R, respectively). IPSC, inhibitory postsynaptic current; WT, wild-type.
Discussion
Many studies have shown that GABAergic inhibition is essential for cholinergically induced oscillations in hippocampus and GABAergic receptor kinetics strongly affects the frequency of these oscillations (Whittington et al. 1995; Fisahn et al. 1998; Mann et al. 2005). Perisomatic synaptic inhibition received by CA3 pyramidal neurons is crucial for hippocampal oscillations in vitro (Mann et al. 2005; Oren et al. 2006). Which GABAA-R subunits mediate this inhibition was not known. It was also not known whether the kinetics of inhibitory synapses on CA3 pyramidal neurons controls the frequency of oscillations in CA1. We find that: (1) hippocampal oscillations are strongly affected in α2−/− mice, but not in α1−/− mice; (2) allosteric modulation of GABAA-Rs containing α2 subunits, but not α1 subunits, affects the kinetics of IPSCs in CA3 pyramidal neurons both during oscillations and by stimulating connected fast spiking interneurons; (3) in CA1 pyramidal neurons, allosteric modulation of GABAA-Rs containing α1 subunits affects the kinetics of IPSCs during oscillations; (4) allosteric modulation of carbachol-induced oscillations in CA3 and CA1 depends mainly on GABAA-Rs with α2 subunits; and (5) only GABAA-Rs located in CA3 affect the frequency of oscillations in hippocampus, and not GABAA-Rs located in CA1. Thus, we conclude that allosteric modulation of GABAA-Rs containing α2 subunits mediates the zolpidem modulation of frequency and power of oscillations. This subunit is expressed at synapses between perisomatic targeting fast spiking interneurons and CA3 pyramidal neurons (Fig. 7).
Figure 7. Schematic view of hippocampal networks involved in oscillations.
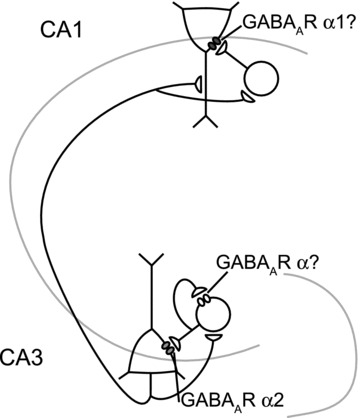
Oscillations are generated in CA3 and depend on feedback inhibition mediated by GABAA receptor α2 subunits. Oscillations in CA1 reflect excitatory inputs from CA3 and feed forward inhibition mediated by GABAA receptor α1 subunits.
Current models on mechanisms underlying the generation of fast network oscillations in hippocampus are based on recurrent excitation and inhibition between pyramidal neurons and interneurons. These models predict that the spike timing and spiking probability of pyramidal neurons, and thereby the synchronization of activity in the hippocampal neuronal network, depends on the decay time of inhibitory inputs (Traub et al. 2000). In line with the models, positive allosteric modulation that lengthens the decay time of IPSCs in CA3 strongly reduces the frequency of oscillations. By increasing and decreasing α subunit function, we assessed which of these α subunits mediates GABAergic inhibition in the recurrent excitation and inhibition in the CA3 region underlying neuronal synchronization. We find that recurrent inhibition is mediated mainly by α2-containing GABAA-Rs, as both IPSCs in CA3 pyramidal neurons and hippocampal network oscillations in α2H101R mice had lost sensitivity to allosteric modulation.
Although allosteric modulation of the α2 subunit had the strongest impact on the frequency of oscillations, other α subunits might also contribute to properties of gamma oscillations. As in α1H101R mice the zolpidem modulation of cholinergically induced oscillations is increased, it is possible that the kinetics of α1 subunit containing receptors regulate the oscillations in opposite direction compared to α2 subunit containing receptors. However, the zolpidem modulation in α2H101R and in α1H101R/α2H101R double mutant was similar, showing that in the α2H101R mice α1 modulation does not affect oscillations. Therefore, we suggest that the modulation of receptors with α1 subunits counteracts the modulatory effects of zolpidem on α2 subunits. The α1 and α2 subunit are the most abundant synaptic subunit in the hippocampus, but α3, α4 and α5 are also expressed (Laurie et al. 1992; Prenosil et al. 2006), although α4 and α5 subunits are not expressed in perisomatic synapses (Nusser et al. 1998; Peng et al. 2002; Fritschy & Brunig, 2003; Prenosil et al. 2006). Furthermore, α4-containing receptors are insensitive to allosteric modulation by zolpidem (Scholze et al. 1996) and would under our experimental conditions not be affected. Deletion of either of these subunits does affect properties of fast network oscillations, probably by changing the excitability of neurons due to a decrease in tonic inhibition (Towers et al. 2004; Mann & Mody, 2010). The α3 subunit is expressed at low abundance in hippocampal pyramidal neurons (Laurie et al. 1992; Prenosil et al. 2006). As this subunit is expressed on perisomatic synapses in the hippocampus (Fritschy et al. 1998), it is possible that the small remaining decrease in frequency by 1 μm zolpidem in α1H101R/α2H101R mice could be accounted by α3-expressing receptors.
Several interneuron types have been shown to fire phase-locked with gamma oscillations in vivo and in vitro (Hajos et al. 2004; Gloveli et al. 2005; Tukker et al. 2007); however, fast spiking basket cells have been shown to be essential for the generation of the cholinergically induced oscillations (Gulyas et al. 2010). We found that zolpidem effects on IPSCs from perisomatic targeting fast spiking interneurons in CA3 are mediated through α2 subunits containing receptors. However, in GABAergic synapses on to pyramidal neurons in CA1 contain more α1 subunits in synapses from parvalbumin-positive basket cells (Klausberger et al. 2002), while synapses from axo-axonic cells and parvalbumin-negative basket cells contain more α2 subunits (Nyiri et al. 2001 but see Kasugai et al. 2010). On the other hand, in benzodiazepine insensitive mice it was shown that synaptic input from stimulations near the soma in CA1 are mediated by α2 containing receptors while stimulations at the distal dendrites activate α1 containing receptors (Prenosil et al. 2006) and the IPSC decay kinetics of synapses from fast spiking basket cells unto pyramidal cells is equal in CA1 and in CA3 (Bartos et al. 2002). Furthermore, it is not known whether the rhythmic GABAergic inhibition in CA1 is mediated via the same interneuron types as in CA3. In CA3 the oscillations depend on local feedback loops (Mann et al. 2005), while in CA1 the rhythmic inhibition is likely coming from CA1 interneurons activated via excitatory input from CA3. We showed that allosteric modulation of IPSCs during oscillations in CA1 pyramidal cells depends on the α1 subunit, indicating that the rhythmic inhibitory input might arrive in different subcellular locations than in CA3 pyramidal cells.
Little is known about which GABAA-R subunits are involved in oscillations in other brain areas. Our findings suggest that GABAA-Rs that contain α2 subunits are important for cholinergically induced fast network oscillations in the hippocampus. Whether these subunits play a similar role in neocortical oscillations is not known. Some indications could come from patients with schizophrenia that show decreased gamma band activity in the frontal cortex (Cho et al. 2006). Pharmacological treatment with GABAA-R agonists that selectively modulate α2/α3-containing receptors increased gamma power in frontal cortical areas and improved cognitive functioning in these patients (Lewis et al. 2008). This may indicate that GABAA-Rs containing α2 subunits may be important for gamma oscillations and cognitive processing in other brain areas as well.
Acknowledgments
We thank Hans Lodder and Rik Jansen for excellent technical assistance, and Charlotte van Coeverden and Sebastian Falk for help with local application experiments. Funding was received from the Netherlands Organization for Scientific Research (NWO; 917.76.360 and 912.06.148), ERC StG ‘BrainSignals’, the Dutch Fund for Economic Structure Reinforcement (FES, 0908 ‘NeuroBasic PharmaPhenomics project’), EU 7th Framework Programme (HEALTH-F2-2009-242167 ‘SynSys’) and VU University Amsterdam.
Author contributions
T.S.H, A.B.B. and H.D.M. designed the experiments. T.S.H., M.R. and A.J.T. performed experiments; T.S.H. analysed the data; T.S.H. and H.D.M. wrote the manuscript. All authors approved the final version.
References
- Atallah BV, Scanziani M. Instantaneous modulation of gamma oscillation frequency by balancing excitation with inhibition. Neuron. 2009;62:566–577. doi: 10.1016/j.neuron.2009.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks MI, Pearce RA. Kinetic differences between synaptic and extrasynaptic GABAA receptors in CA1 pyramidal cells. J Neurosci. 2000;20:937–948. doi: 10.1523/JNEUROSCI.20-03-00937.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartos M, Vida I, Frotscher M, Meyer A, Monyer H, Geiger JR, Jonas P. Fast synaptic inhibition promotes synchronized gamma oscillations in hippocampal interneuron networks. Proc Natl Acad Sci U S A. 2002;99:13 222–13 227. doi: 10.1073/pnas.192233099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm SL, 2nd, Ponomarev I, Jennings AW, Whiting PJ, Rosahl TW, Garrett EM, Blednov YA, Harris RA. γ-Aminobutyric acid A receptor subunit mutant mice: new perspectives on alcohol actions. Biochem Pharmacol. 2004;68:1581–1602. doi: 10.1016/j.bcp.2004.07.023. [DOI] [PubMed] [Google Scholar]
- Bosman LW, Heinen K, Spijker S, Brussaard AB. Mice lacking the major adult GABAA receptor subtype have normal number of synapses, but retain juvenile IPSC kinetics until adulthood. J Neurophysiol. 2005;94:338–346. doi: 10.1152/jn.00084.2005. [DOI] [PubMed] [Google Scholar]
- Bragin A, Jando G, Nadasdy Z, Hetke J, Wise K, Buzsaki G. Gamma (40–100 Hz) oscillation in the hippocampus of the behaving rat. J Neurosci. 1995;15:47–60. doi: 10.1523/JNEUROSCI.15-01-00047.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardin JA, Carlen M, Meletis K, Knoblich U, Zhang F, Deisseroth K, Tsai LH, Moore CI. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009;459:663–667. doi: 10.1038/nature08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho RY, Konecky RO, Carter CS. Impairments in frontal cortical gamma synchrony and cognitive control in schizophrenia. Proc Natl Acad Sci U S A. 2006;103:19 878–19 883. doi: 10.1073/pnas.0609440103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope DW, Halbsguth C, Karayannis T, Wulff P, Ferraguti F, Hoeger H, Leppa E, Linden AM, Oberto A, Ogris W, Korpi ER, Sieghart W, Somogyi P, Wisden W, Capogna M. Loss of zolpidem efficacy in the hippocampus of mice with the GABAA receptor γ2 F77I point mutation. Eur J Neurosci. 2005;21:3002–3016. doi: 10.1111/j.1460-9568.2005.04127.x. [DOI] [PubMed] [Google Scholar]
- Csicsvari J, Jamieson B, Wise KD, Buzsaki G. Mechanisms of gamma oscillations in the hippocampus of the behaving rat. Neuron. 2003;37:311–322. doi: 10.1016/s0896-6273(02)01169-8. [DOI] [PubMed] [Google Scholar]
- Dias R, Sheppard WF, Fradley RL, Garrett EM, Stanley JL, Tye SJ, Goodacre S, Lincoln RJ, Cook SM, Conley R, Hallett D, Humphries AC, Thompson SA, Wafford KA, Street LJ, Castro JL, Whiting PJ, Rosahl TW, Atack JR, McKernan RM, Dawson GR, Reynolds DS. Evidence for a significant role of α3-containing GABAA receptors in mediating the anxiolytic effects of benzodiazepines. J Neurosci. 2005;25:10 682–10 688. doi: 10.1523/JNEUROSCI.1166-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson R, Awaiz S, Whittington MA, Lieb WR, Franks NP. The effects of general anaesthetics on carbachol-evoked gamma oscillations in the rat hippocampus in vitro. Neuropharmacology. 2003;44:864–872. doi: 10.1016/s0028-3908(03)00083-2. [DOI] [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters in The Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisahn A, Pike FG, Buhl EH, Paulsen O. Cholinergic induction of network oscillations at 40 Hz in the hippocampus in vitro. Nature. 1998;394:186–189. doi: 10.1038/28179. [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Brunig I. Formation and plasticity of GABAergic synapses: physiological mechanisms and pathophysiological implications. Pharmacol Ther. 2003;98:299–323. doi: 10.1016/s0163-7258(03)00037-8. [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Weinmann O, Wenzel A, Benke D. Synapse-specific localization of NMDA and GABAA receptor subunits revealed by antigen-retrieval immunohistochemistry. J Comp Neurol. 1998;390:194–210. [PubMed] [Google Scholar]
- Fuchs EC, Zivkovic AR, Cunningham MO, Middleton S, Lebeau FE, Bannerman DM, Rozov A, Whittington MA, Traub RD, Rawlins JN, Monyer H. Recruitment of parvalbumin-positive interneurons determines hippocampal function and associated behavior. Neuron. 2007;53:591–604. doi: 10.1016/j.neuron.2007.01.031. [DOI] [PubMed] [Google Scholar]
- Gloveli T, Dugladze T, Saha S, Monyer H, Heinemann U, Traub RD, Whittington MA, Buhl EH. Differential involvement of oriens/pyramidale interneurones in hippocampal network oscillations in vitro. J Physiol. 2005;562:131–147. doi: 10.1113/jphysiol.2004.073007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulyas AI, Szabo GG, Ulbert I, Holderith N, Monyer H, Erdelyi F, Szabo G, Freund TF, Hajos N. Parvalbumin-containing fast-spiking basket cells generate the field potential oscillations induced by cholinergic receptor activation in the hippocampus. J Neurosci. 2010;30:15134–15145. doi: 10.1523/JNEUROSCI.4104-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajos N, Palhalmi J, Mann EO, Nemeth B, Paulsen O, Freund TF. Spike timing of distinct types of GABAergic interneuron during hippocampal gamma oscillations in vitro. J Neurosci. 2004;24:9127–9137. doi: 10.1523/JNEUROSCI.2113-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heistek TS, Timmerman AJ, Spijker S, Brussaard AB, Mansvelder HD. GABAergic synapse properties may explain genetic variation in hippocampal network oscillations in mice. Front Cell Neurosci. 2010;4:18. doi: 10.3389/fncel.2010.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horikawa K, Armstrong WE. A versatile means of intracellular labeling: injection of biocytin and its detection with avidin conjugates. J Neurosci Methods. 1988;25:1–11. doi: 10.1016/0165-0270(88)90114-8. [DOI] [PubMed] [Google Scholar]
- Hutcheon B, Morley P, Poulter MO. Developmental change in GABAA receptor desensitization kinetics and its role in synapse function in rat cortical neurons. J Physiol. 2000;522:3–17. doi: 10.1111/j.1469-7793.2000.t01-5-00003.xm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen R, Timmerman J, Loos M, Spijker S, van Ooyen A, Brussaard AB, Mansvelder HD, Smit AB, de Gunst M, Linkenkaer-Hansen K. Novel candidate genes associated with hippocampal oscillations. PLoS One. 2011;6:e26586. doi: 10.1371/journal.pone.0026586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasugai Y, Swinny JD, Roberts JD, Dalezios Y, Fukazawa Y, Sieghart W, Shigemoto R, Somogyi P. Quantitative localisation of synaptic and extrasynaptic GABAA receptor subunits on hippocampal pyramidal cells by freeze-fracture replica immunolabelling. Eur J Neurosci. 2010;32:1868–1888. doi: 10.1111/j.1460-9568.2010.07473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausberger T, Roberts JD, Somogyi P. Cell type- and input-specific differences in the number and subtypes of synaptic GABAA receptors in the hippocampus. J Neurosci. 2002;22:2513–2521. doi: 10.1523/JNEUROSCI.22-07-02513.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausberger T, Magill PJ, Marton LF, Roberts JD, Cobden PM, Buzsaki G, Somogyi P. Brain-state- and cell-type-specific firing of hippocampal interneurons in vivo. Nature. 2003;421:844–848. doi: 10.1038/nature01374. [DOI] [PubMed] [Google Scholar]
- Laurie DJ, Wisden W, Seeburg PH. The distribution of thirteen GABAA receptor subunit mRNAs in the rat brain. III. Embryonic and postnatal development. J Neurosci. 1992;12:4151–4172. doi: 10.1523/JNEUROSCI.12-11-04151.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Cho RY, Carter CS, Eklund K, Forster S, Kelly MA, Montrose D. Subunit-selective modulation of GABA type A receptor neurotransmission and cognition in schizophrenia. Am J Psychiatry. 2008;165:1585–1593. doi: 10.1176/appi.ajp.2008.08030395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman JE, Idiart MA. Storage of 7 +/− 2 short-term memories in oscillatory subcycles. Science. 1995;267:1512–1515. doi: 10.1126/science.7878473. [DOI] [PubMed] [Google Scholar]
- Mann EO, Mody I. Control of hippocampal gamma oscillation frequency by tonic inhibition and excitation of interneurons. Nat Neurosci. 2010;13:205–212. doi: 10.1038/nn.2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann EO, Suckling JM, Hajos N, Greenfield SA, Paulsen O. Perisomatic feedback inhibition underlies cholinergically induced fast network oscillations in the rat hippocampus in vitro. Neuron. 2005;45:105–117. doi: 10.1016/j.neuron.2004.12.016. [DOI] [PubMed] [Google Scholar]
- McKernan RM, Rosahl TW, Reynolds DS, Sur C, Wafford KA, Atack JR, Farrar S, Myers J, Cook G, Ferris P, Garrett L, Bristow L, Marshall G, Macaulay A, Brown N, Howell O, Moore KW, Carling RW, Street LJ, Castro JL, Ragan CI, Dawson GR, Whiting PJ. Sedative but not anxiolytic properties of benzodiazepines are mediated by the GABAA receptor α1 subtype. Nat Neurosci. 2000;3:587–592. doi: 10.1038/75761. [DOI] [PubMed] [Google Scholar]
- Montgomery SM, Buzsaki G. Gamma oscillations dynamically couple hippocampal CA3 and CA1 regions during memory task performance. Proc Natl Acad Sci U S A. 2007;104:14495–14500. doi: 10.1073/pnas.0701826104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusser Z, Sieghart W, Somogyi P. Segregation of different GABAA receptors to synaptic and extrasynaptic membranes of cerebellar granule cells. J Neurosci. 1998;18:1693–1703. doi: 10.1523/JNEUROSCI.18-05-01693.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyiri G, Freund TF, Somogyi P. Input-dependent synaptic targeting of α2-subunit-containing GABAA receptors in synapses of hippocampal pyramidal cells of the rat. Eur J Neurosci. 2001;13:428–442. doi: 10.1046/j.1460-9568.2001.01407.x. [DOI] [PubMed] [Google Scholar]
- Oren I, Mann EO, Paulsen O, Hajos N. Synaptic currents in anatomically identified CA3 neurons during hippocampal gamma oscillations in vitro. J Neurosci. 2006;26:9923–9934. doi: 10.1523/JNEUROSCI.1580-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oren I, Hajos N, Paulsen O. Identification of the current generator underlying cholinergically induced gamma frequency field potential oscillations in the hippocampal CA3 region. J Physiol. 2010;588:785–797. doi: 10.1113/jphysiol.2009.180851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palhalmi J, Paulsen O, Freund TF, Hajos N. Distinct properties of carbachol- and DHPG-induced network oscillations in hippocampal slices. Neuropharmacology. 2004;47:381–389. doi: 10.1016/j.neuropharm.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Peng Z, Hauer B, Mihalek RM, Homanics GE, Sieghart W, Olsen RW, Houser CR. GABAA receptor changes in delta subunit-deficient mice: altered expression of α4 and γ2 subunits in the forebrain. J Comp Neurol. 2002;446:179–197. doi: 10.1002/cne.10210. [DOI] [PubMed] [Google Scholar]
- Prenosil GA, Schneider Gasser EM, Rudolph U, Keist R, Fritschy JM, Vogt KE. Specific subtypes of GABAA receptors mediate phasic and tonic forms of inhibition in hippocampal pyramidal neurons. J Neurophysiol. 2006;96:846–857. doi: 10.1152/jn.01199.2005. [DOI] [PubMed] [Google Scholar]
- Pritchett DB, Seeburg PH. Gamma-aminobutyric acidA receptor α5-subunit creates novel type II benzodia-zepine receptor pharmacology. J Neurochem. 1990;54:1802–1804. doi: 10.1111/j.1471-4159.1990.tb01237.x. [DOI] [PubMed] [Google Scholar]
- Rudolph U, Crestani F, Benke D, Brunig I, Benson JA, Fritschy JM, Martin JR, Bluethmann H, Mohler H. Benzodiazepine actions mediated by specific γ-aminobutyric acidA receptor subtypes. Nature. 1999;401:796–800. doi: 10.1038/44579. [DOI] [PubMed] [Google Scholar]
- Scholze P, Ebert V, Sieghart W. Affinity of various ligands for GABAA receptors containing α4β3γ2, α4γ2, or α1β3γ2 subunits. Eur J Pharmacol. 1996;304:155–162. doi: 10.1016/0014-2999(96)00088-x. [DOI] [PubMed] [Google Scholar]
- Sur C, Wafford KA, Reynolds DS, Hadingham KL, Bromidge F, Macaulay A, Collinson N, O’Meara G, Howell O, Newman R, Myers J, Atack JR, Dawson GR, McKernan RM, Whiting PJ, Rosahl TW. Loss of the major GABAA receptor subtype in the brain is not lethal in mice. J Neurosci. 2001;21:3409–3418. doi: 10.1523/JNEUROSCI.21-10-03409.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towers SK, Gloveli T, Traub RD, Driver JE, Engel D, Fradley R, Rosahl TW, Maubach K, Buhl EH, Whittington MA. α5 subunit-containing GABAA receptors affect the dynamic range of mouse hippocampal kainate-induced gamma frequency oscillations in vitro. J Physiol. 2004;559:721–728. doi: 10.1113/jphysiol.2004.071191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub RD, Bibbig A, Fisahn A, LeBeau FE, Whittington MA, Buhl EH. A model of gamma-frequency network oscillations induced in the rat CA3 region by carbachol in vitro. Eur J Neurosci. 2000;12:4093–4106. doi: 10.1046/j.1460-9568.2000.00300.x. [DOI] [PubMed] [Google Scholar]
- Tukker JJ, Fuentealba P, Hartwich K, Somogyi P, Klausberger T. Cell type-specific tuning of hippocampal interneuron firing during gamma oscillations in vivo. J Neurosci. 2007;27:8184–8189. doi: 10.1523/JNEUROSCI.1685-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicini S, Ferguson C, Prybylowski K, Kralic J, Morrow AL, Homanics GE. GABAA receptor α1 subunit deletion prevents developmental changes of inhibitory synaptic currents in cerebellar neurons. J Neurosci. 2001;21:3009–3016. doi: 10.1523/JNEUROSCI.21-09-03009.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittington MA, Traub RD, Jefferys JG. Synchronized oscillations in interneuron networks driven by metabotropic glutamate receptor activation. Nature. 1995;373:612–615. doi: 10.1038/373612a0. [DOI] [PubMed] [Google Scholar]


