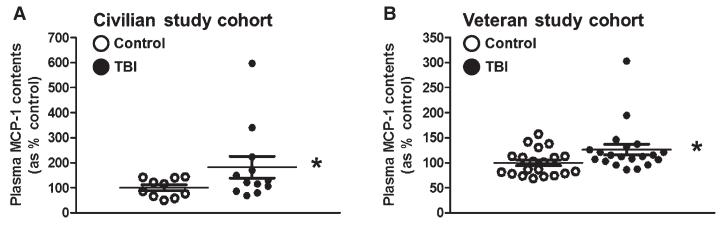
Fig. 2.
Validation of plasma MCP-1 content as a clinically accessible TBI biomarker in two demographically distinct TBI study cohorts. An independent, quantitative ELISA assay was used to assess plasma MCP-1 contents in TBI and control cases from a civilian and a military veteran study cohort. (A) Plasma MCP-1 contents in TBI (n = 14) and control (n = 10) cases from the civilian study cohort (Table 1). (B) Plasma MCP-1 contents among veteran TBI (n = 20) and control (n = 20) cases (Table 2). Scatter graphs represent values for individual cases and group mean values ± SEM of plasma biomarker contents, expressed as % of controls. *p < 0.05 by student t-test, TBI compared to the control group.