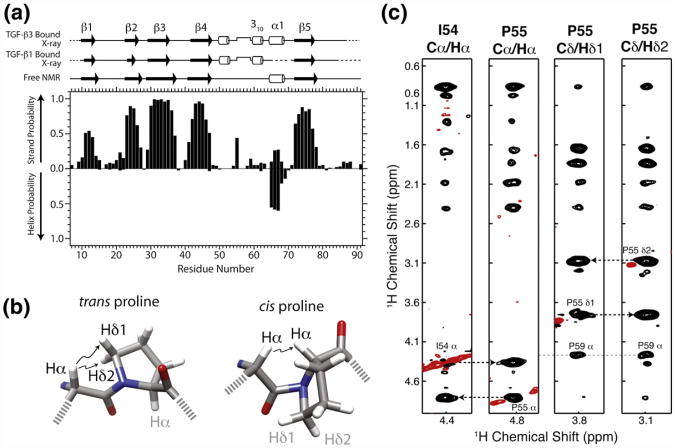
Fig. 3.
TβR-I alone adopts a similar overall secondary structure and cis-prolyl peptide bond compared to the bound form. (a) Secondary structural probabilities for the unbound form of TβR-I, deduced on the basis of secondary shifts using the program PECAN,24 correlate closely with secondary structures for the TGF-β1-bound and TGF-β3-bound forms of TβR-I (PDB codes: 3KFD and 2PJY, respectively). Secondary structures were calculated from the structures of the bound forms using the program DSSP.25 (b) cis-Xaa-Pro and trans-Xaa-Pro peptide bonds are characterized by close interproton distances between Xaa Hα and either Pro Hα or Pro Hδ1/Hδ2, respectively.26 (c) Strips from a 3D 13C-edited NOESY spectrum from the Cα/Hα positions of Ile54 Hα, Pro55 Hα, Pro55 Hδ1, and Pro55 H δ2. NOEs between Ile54 Hα and Pro55 Hα indicative of a cis peptide bond are identified by broken lines. Positive and negative signals are drawn with black and red contours, respectively.