Abstract
Aims/hypothesis
Plasma ceramide concentrations correlate with insulin sensitivity, inflammation and atherosclerotic risk. We hypothesised that plasma ceramide concentrations are increased in the presence of elevated fatty acid levels and are regulated by increased liver serine C-palmitoyltransferase (SPT) activity.
Methods
Lean humans and rats underwent an acute lipid infusion and plasma ceramide levels were determined. One group of lipid-infused rats was administered myriocin to inhibit SPT activity. Liver SPT activity was determined in lipid-infused rats, and obese, insulin resistant mice. The time and palmitate dose-dependent synthesis of intracellular and secreted ceramide was determined in HepG2 liver cells.
Results
Plasma ceramide levels were increased during lipid infusion in humans and rats, and in obese, insulin-resistant mice. The increase in plasma ceramide was not associated with changes in liver SPT activity, and inhibiting SPT activity by ~50% did not alter plasma ceramide levels in lipid-infused rats. In HepG2 liver cells, palmitate incorporation into extracellular ceramide was both dose- and time-dependent, suggesting the liver cells rapidly secreted the newly synthesised ceramide.
Conclusions/interpretation
Elevated systemic fatty acid availability increased plasma ceramide but this was not associated with changes in hepatic SPT activity, suggesting that liver ceramide synthesis is driven by substrate availability rather than increased SPT activity. This report also provides evidence that the liver is sensitive to the intracellular ceramide concentration, and an increase in liver ceramide secretion may help protect the liver from the deleterious effects of intracellular ceramide accumulation.
Keywords: De novo ceramide synthesis, Ex vivo SPT activity, HepG2 liver cells, Lipid infusion, Liver, Myriocin
Introduction
Several mechanism(s) have been proposed to explain how excess lipid causes insulin resistance. One such hypothesis states that the sphingolipid ceramide accumulates in insulin-sensitive tissues such as skeletal muscle and liver to inhibit insulin signal transduction [1]. Recent observations have expanded on this paradigm and report increases in plasma ceramide in obesity that correlate with the severity of insulin resistance, inflammation and atherosclerotic risk [2–4].
The liver is considered a primary source of plasma ceramides, as evidenced by the close overlap in the ceramide species profiles of the liver and plasma [2], the fact that 75% of ceramides are contained in VLDL or LDL [5] and that ceramides secreted by isolated hepatocytes are largely contained in VLDL [6]. Ceramide is generated through a number of pathways, including de novo synthesis from long-chain fatty acids, from the hydrolysis of the membrane phospholipid sphingomyelin and via the salvage pathway (for a review, see [1]). The major pathway for ceramide synthesis from fatty acids is the de novo synthesis pathway, and the regulation of flux through this pathway is thought to link ceramide accumulation and insulin resistance arising from excess fatty acids [7]. The first committed step of the de novo pathway is the condensation of L-serine and palmitoyl-CoA by the rate-limiting enzyme, serine C-palmitoyltransferase (SPT; EC 2.3.1.50). Pharmacological inhibition of SPT prevents ceramide accumulation and restores insulin action, placing SPT at the centre of potential therapies to treat insulin resistance [7, 8]. However, most studies to date have not actually measured SPT activity, but rather have determined the expression of the genes encoding the SPT subunits and inferred changes in transferase activity [9, 10].
The aims of this study were to establish whether increased availability of plasma fatty acids is sufficient to increase plasma ceramide levels and to determine whether liver SPT activity mediates changes in plasma ceramides.
Methods
Acute fatty acid oversupply in vivo
Human lipid infusion
The University of Michigan Institutional Review Board approved all experimental procedures and participants provided written informed consent. Seven healthy young women (mean±SEM: age 27±4 years; BMI 23±1 kg/m2) participated in two experimental trials that were randomised and separated by at least 7 days. Participants were infused overnight (16 h) with saline (control; 0.55 ml kg−1 h−1) or lipid (Liposyn; Abbott Laboratories, Abbott Park, IL; 0.55 ml kg−1 h−1) and heparin (5 U kg−1 h−1) to increase plasma NEFA concentrations to a high physiological level (~1.0 mmol/l). Further details of this experiment are described in the electronic supplementary material (ESM) Methods.
Rat lipid infusion
The Monash University School of Biomedical Sciences Animal Ethics Committee approved all procedures, and the Principles of Laboratory Animal Care were followed. Adult male Wistar rats (Animal Resources Centre, Perth, WA, Australia) with indwelling catheters placed into the right and left jugular veins were deprived of food for 5 h overnight. The lipid-infused group received 7% (vol./vol.) Intralipid (Baxter Healthcare, Sydney, NSW, Australia; 1.7 ml/h) with heparin infusion (40 U/h) and control animals were infused with 2.5% glycerol. The ceramide content in the Intralipid emulsion was 88 nmol/l, thus the ceramide infusion rate was 52 pmol/h. Both groups were infused for 5 h and rats killed (Lethabarb; Virbac Animal Health, Sydney, Australia) at the end of the infusion (5 h group) or 2 h (7 h) or 7 h (12 h) after the cessation of the infusion. In a separate experiment, rats were administered 100 μg/kg i.v. of myriocin (Sigma, Castle Hill, NSW, Australia) 5 min prior to the infusion protocol. Tissue and plasma samples were collected at the end of the 5 h infusion. Further details are provided in the ESM Methods.
In vitro ceramide synthesis and secretion
HepG2 cells were grown in DMEM supplemented with 5 mmol/l glucose, 10% (vol./vol.) fetal bovine serum and 1% penicillin/streptomycin. Assay medium was prepared from stock fatty acid solutions (100 mmol/l palmitate dissolved in 100% ethanol) conjugated to 2% (wt/vol.) fatty acid-free BSA in DMEM with 5 mmol/l glucose, 0.4 mmol/l L-serine and no antibiotics. The duration and concentration of palmitate treatment are detailed in the related figure legend. For assessment of radiolabelled L-serine incorporation into ceramide, the assay media contained 0.5 mmol/l palmitate, 0.4 mmol/l L-serine, and 1.11 MBq/ml L-[3H]serine (Perkin Elmer, Glen Waverley, VIC, Australia) and cells were incubated for 4 or 8 h. Further details of this experiment can be found in the ESM Methods.
Analytical methods
Whole blood was centrifuged at 14,000 g for 90 s, and plasma was analysed to determine free fatty acids (NEFA-C, Wako Pure Chemical Industries, Osaka, Japan). Plasma (150 μl) and tissue (10 mg) total ceramide were extracted and quantified based on published methods [11] with minor modifications [12]. Plasma ceramide species were determined as previously described [13]. Serine C-palmitoyltransferase activity was assessed as previously described [14] and further details can be found in the ESM Methods.
Quantitative real-time PCR
RNA was extracted from whole liver using Qiazol and 1 μg RNA was reverse transcribed (iScript cDNA Synthesis Kit, BioRad Laboratories, Hercules, CA, USA). Quantitative real-time PCR was performed on a realplex Mastercycler (Eppendorf, North Ryde, NSW, Australia) using the TaqMan Universal PCR Master Mix and TaqMan Gene Expression Assays (ESM Table 1; Applied Biosystems, Foster City, CA, USA). The relative quantification was calculated using the ΔΔCt method, normalising values to the appropriate control group.
Statistical analysis
Statistical analyses were performed with the use of a statistics package (Graphpad Prism5; Graphpad Software, San Diego, CA, USA). Differences among relevant groups were assessed using unpaired Student’s t test, linear regression analysis, one-way ANOVA or two-way ANOVA with Tukey–Kramer post hoc tests as appropriate and noted in figure legends. A p value ≤0.05 was considered significant. Data are reported as mean±SEM.
Results
Increasing fatty acid availability increases plasma ceramide levels in humans and rats
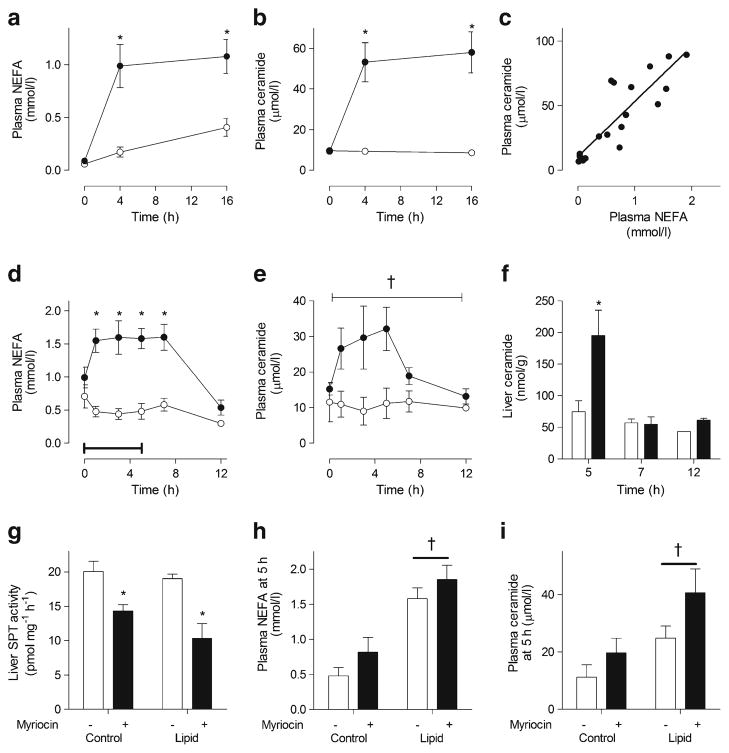
Lipid infusion in humans increased plasma NEFA (Fig. 1a) and plasma ceramide levels (Fig. 1b). Plasma NEFA and ceramide concentrations were tightly correlated during the lipid infusion (Fig. 1c). A concomitant increase in plasma NEFA (Fig. 1d) and plasma ceramide (Fig. 1e) levels also occurred during the 5 h lipid infusion in rats. The timing of the transient elevation in plasma ceramide closely matched that of liver ceramide levels (Fig. 1f), which returned to pre-infusion levels within 2 h after cessation of the lipid infusion. There was no change in liver SPT activity in lipid-infused rats (ESM Fig. 1). As mentioned in the Methods, the ceramide infusion rate was 52 pmol/h, which cannot explain the increase in plasma ceramide levels of 15 μmol/l. Taken together, these results suggest that plasma NEFAs increase liver ceramide levels and plasma ceramide secretion, independently of SPT.
Fig. 1.
Lipid infusion increases plasma NEFA and ceramide levels. (a, b) plasma NEFA and ceramide levels in humans infused with either saline (control, white) or lipid and heparin (lipid, black) *p<0.05 vs control (by two-way ANOVA); n=7 per group. (c) Linear regression analysis of NEFA and ceramide levels in humans infused with lipid and heparin. R2=0.75, p<0.0001. (d, e) plasma NEFA and ceramide levels in rats infused with either 2.5% glycerol (control, white) or lipid and heparin (lipid, black) for 5 h. Bar represents the duration of infusion. (f) Liver ceramide content, *p<0.05 vs control, †p<0.05 main effect of infusion (by two-way ANOVA), n=4–14. (g) Maximal ex vivo liver SPT activity, (h) plasma NEFA, (i) ceramide levels in rats infused with either 2.5% glycerol (control) or lipid and heparin (lipid) for 5 h, administered 100 μg/kg myriocin i.v. 5 min prior to infusion (black) or saline (white). *p<0.05 vs no myriocin; †p<0.05 main effect of infusion; n=5–8 per group. Values are mean±SEM
Reducing SPT activity does not alter plasma ceramide level in lipid-infused rats
We examined the role of SPT in modulating NEFA-induced plasma ceramide production by blocking its activity with myriocin. While myriocin reduced liver SPT activity by 28–46% in rats (Fig. 1g), this did not affect circulating NEFA or ceramide levels (Fig. 1h,i). These data argue that reducing SPT activity by up to 50% does not affect NEFA-mediated increases in plasma ceramides.
SPT activity is not altered in models of chronically elevated fatty acid availability
In mice, high-fat feeding increased plasma ceramide levels (ESM Fig. 2a) but did not affect liver ceramide content (ESM Fig. 2b). Targeted analysis of ceramide metabolism genes showed increased Sptlc2 expression with high-fat feeding (ESM Fig. 2c), yet SPT activity was unaffected (ESM Fig. 2d). Similarly, plasma NEFA levels (ESM Fig. 3a) and liver and plasma ceramide levels (ESM Fig. 3b,c) were increased in obese, hyperinsulinaemic ob/ob mice compared with lean controls. These changes were accompanied by increased expression of the genes for the liver SPT subunits (ESM Fig. 3d), but no increase in SPT activity (ESM Fig. 3e). These data reinforce the hypothesis that plasma ceramide and liver SPT activity are disconnected in murine models of obesity-associated fatty acid oversupply.
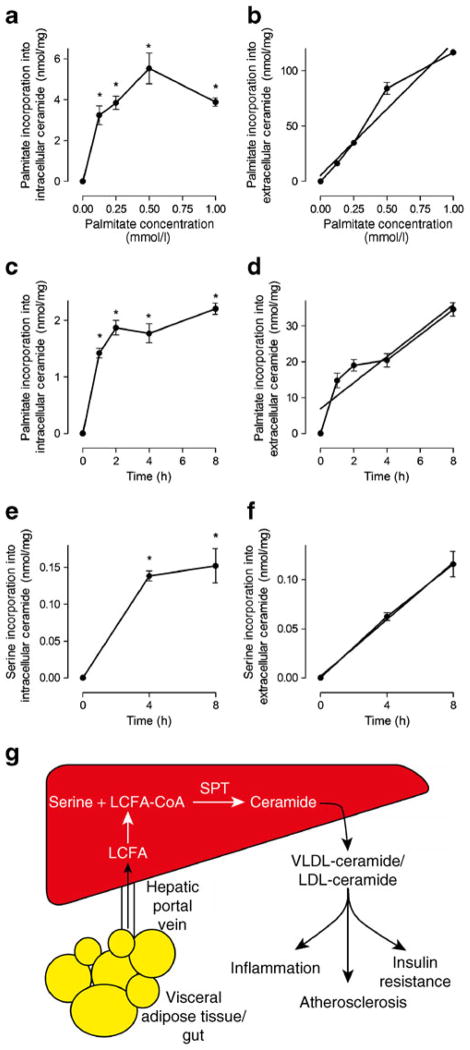
Fig. 2.
Dose and time course of incorporation of palmitate or serine into intracellular or extracellular ceramide in HepG2 cells. (a, b) Dose–response for incorporation of palmitate into intracellular or extracellular ceramide. (c, d) Time course of incorporation of palmitate into intracellular or extracellular ceramide. Cells were incubated with 0.5 mmol/l cold palmitate and 0.4 mmol/l cold serine. (e, f) Time course of incorporation of L-serine into intracellular or extracellular ceramide. Cells were incubated with 0.5 mmol/l cold palmitate and 0.4 mmol/l cold L-serine. Linear regression analysis was performed: (b) R2=0.94, p<0.0001, (d) R2=0.81, p<0.0001, (f) R2=0.95, p< 0.0001. Values are mean±SEM. *p<0.05 vs t=0 (by one-way ANOVA), n=3 per group. (g) Schematic diagram of proposed model whereby in models of increased long-chain fatty acid (LCFA) availability, activated LCFA (LCFA-CoA) is converted into ceramide via de novo ceramide synthesis and then packaged into VLDL and LDL, which has been associated with various pathologies
Intracellular de novo ceramide synthesis increases ceramide secretion in HepG2 liver cells
Palmitate incorporation into intracellular ceramide in HepG2 cells increased markedly above control levels across a broad range of concentrations (Fig. 2a). According to 1-way ANOVA, concentrations above 0.125 mmol/l did not further augment the incorporation of palmitate into the intracellular ceramide pool (Fig. 2a). This was attributed to an increase in ceramide secretion, which increased linearly as a function of palmitate concentration (Fig. 2b). In time course experiments, the amount of palmitate incorporated into intracellular ceramide plateaued after 1 h of incubation and remained elevated thereafter (Fig. 2c). By contrast, palmitate incorporation into extracellular ceramide increased linearly with time (Fig. 2d), suggesting that the newly synthesised ceramide was secreted from liver cells. Similar patterns of de novo ceramide production and secretion were apparent when radiolabelled L-serine was used as the SPT substrate (Fig. 2e,f). Collectively, these data suggest that HepG2 liver cells secrete ceramide in a dose- and time-dependent manner that is sensitive to intracellular de novo ceramide synthesis.
Discussion
Ceramide accumulation in ectopic tissues promotes insulin resistance, and recent studies have proposed that plasma ceramides cause insulin resistance and inflammation and increase atherosclerotic risk [2–4, 9]. The factors mediating the increase in plasma ceramides in obesity are not well described and we sought to determine whether increasing fatty acid flux, which is a characteristic of obesity and type 2 diabetes, modulates plasma ceramide levels. We provide evidence that increasing fatty acid availability is accompanied by an increase in plasma ceramide levels in several independent experimental models, including lipid and heparin infusion in humans and rats, high-fat fed mice and ob/ob mice and cultured liver cells. We showed in vivo that an increase in fatty acid availability not only increased liver ceramide content, but also increased plasma ceramide concentrations over a similar time course, indicating that ceramide secretion was upregulated with ceramide production. In line with this interpretation, our cell-based studies showed a close relationship between intracellular de novo ceramide production and ceramide secretion. On the basis of these complementary observations, we propose that the liver detects intracellular ceramide levels with precision, and increases ceramide secretion, which may protect the liver from the pathogenic effects of intracellular ceramide accumulation (Fig. 2g). Thus, further investigations examining liver ceramide synthesis and packaging into lipoproteins are warranted, as are further studies that delineate the potential role of plasma ceramides in metabolic diseases.
We unexpectedly found that SPT enzyme activity was not elevated in obese, insulin-resistant mice that have increased plasma ceramide levels, and that reducing liver SPT activity by ~50% did not alter these levels. These observations question the role of SPT as a rate-limiting factor for acutely regulating flux through the de novo ceramide synthesis pathway in patho/physiological settings and highlight that interventions modulating hepatic SPT activity may be unlikely to reduce plasma ceramide concentrations in common chronic diseases associated with insulin resistance and type 2 diabetes.
Acknowledgments
Funding These studies were supported in part by research grants from the National Health and Medical Research Council (NHMRC) of Australia and a Monash University Fellowship, and the National Institutes of Health. A.J. Hoy is supported by an NHMRC Biomedical Australian Training Fellowship, C.R. Bruce an NHMRC Career Development Award, and M.J. Watt by a NHMRC Senior Research Fellowship. J.F. Horowitz is supported by grants from the National Institutes of Health (NIH; R01 DK077966 and P30-DK-089503). S. Schenk is supported by grants from the NIH (R24 HD050837, P30 AR058878).
Abbreviations
- HFD
High-fat diet
- LFD
Low-fat diet
- SPT
Serine C-palmitoyltransferase
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00125-012-2649-3) contains peer-reviewed but unedited supplementary material, which is available to authorised users.
Duality of interest The authors declare that there is no duality of interest associated with this manuscript.
Contribution statement AJH was responsible for the conception and design of the study, analysis and interpretation of data, design of the article and drafting the article. MJW was responsible for the conception and design of the study, analysis and interpretation of data and revised the manuscript critically. ACB, CRB, SS and JFH were responsible for analysis and interpretation of data and revised the manuscript. All authors gave final approval.
Contributor Information
M. J. Watt, Email: matthew.watt@monash.edu, Department of Physiology, Monash University, Building 13F, Clayton, VIC 3800, Australia
A. C. Barnett, Department of Physiology, Monash University, Building 13F, Clayton, VIC 3800, Australia
C. R. Bruce, Department of Physiology, Monash University, Building 13F, Clayton, VIC 3800, Australia. Baker IDI Heart & Diabetes Institute, Melbourne, VIC, Australia
S. Schenk, School of Kinesiology, University of Michigan, Ann Arbor, MI, USA. Department of Orthopaedic Surgery, University of California, San Diego, CA, USA
J. F. Horowitz, School of Kinesiology, University of Michigan, Ann Arbor, MI, USA
A. J. Hoy, Email: andrew.hoy@sydney.edu.au, Department of Physiology, Monash University, Building 13F, Clayton, VIC 3800, Australia. Discipline of Physiology, University of Sydney, Anderson Stuart Building (F13), Sydney, NSW 2006, Australia
References
- 1.Lipina C, Hundal HS. Sphingolipids: agents provocateurs in the pathogenesis of insulin resistance. Diabetologia. 2011;54:1596–1607. doi: 10.1007/s00125-011-2127-3. [DOI] [PubMed] [Google Scholar]
- 2.Haus JM, Kashyap SR, Kasumov T, et al. Plasma ceramides are elevated in obese subjects with type 2 diabetes and correlate with the severity of insulin resistance. Diabetes. 2009;58:337–343. doi: 10.2337/db08-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Mello VD, Lankinen M, Schwab U, et al. Link between plasma ceramides, inflammation and insulin resistance: association with serum IL-6 concentration in patients with coronary heart disease. Diabetologia. 2009;52:2612–2615. doi: 10.1007/s00125-009-1482-9. [DOI] [PubMed] [Google Scholar]
- 4.Ichi I, Nakahara K, Miyashita Y, et al. Association of ceramides in human plasma with risk factors of atherosclerosis. Lipids. 2006;41:859–863. doi: 10.1007/s11745-006-5041-6. [DOI] [PubMed] [Google Scholar]
- 5.Wiesner P, Leidl K, Boettcher A, Schmitz G, Liebisch G. Lipid profiling of FPLC-separated lipoprotein fractions by electrospray ionization tandem mass spectrometry. J Lipid Res. 2009;50:574–585. doi: 10.1194/jlr.D800028-JLR200. [DOI] [PubMed] [Google Scholar]
- 6.Merrill AH, Jr, Lingrell S, Wang E, Nikolova-Karakashian M, Vales TR, Vance DE. Sphingolipid biosynthesis de novo by rat hepatocytes in culture. Ceramide and sphingomyelin are associated with, but not required for, very low density lipoprotein secretion. J Biol Chem. 1995;270:13834–13841. doi: 10.1074/jbc.270.23.13834. [DOI] [PubMed] [Google Scholar]
- 7.Holland WL, Brozinick JT, Wang LP, et al. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab. 2007;5:167–179. doi: 10.1016/j.cmet.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 8.Ussher JR, Koves TR, Cadete VJ, et al. Inhibition of de novo ceramide synthesis reverses diet-induced insulin resistance and enhances whole-body oxygen consumption. Diabetes. 2010;59:2453–2464. doi: 10.2337/db09-1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Samad F, Hester KD, Yang G, Hannun YA, Bielawski J. Altered adipose and plasma sphingolipid metabolism in obesity: a potential mechanism for cardiovascular and metabolic risk. Diabetes. 2006;55:2579–2587. doi: 10.2337/db06-0330. [DOI] [PubMed] [Google Scholar]
- 10.Lyn-Cook LE, Jr, Lawton M, Tong M, et al. Hepatic ceramide may mediate brain insulin resistance and neurodegeneration in Diabetologia type 2 diabetes and non-alcoholic steatohepatitis. J Alzheimers Dis. 2009;16:715–729. doi: 10.3233/JAD-2009-0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Preiss J, Loomis CR, Bishop WR, Stein R, Niedel JE, Bell RM. Quantitative measurement of sn-1,2-diacylglycerols present in platelets, hepatocytes, and ras- and sis-transformed normal rat kidney cells. J Biol Chem. 1986;261:8597–8600. [PubMed] [Google Scholar]
- 12.Watt MJ, Hevener A, Lancaster GI, Febbraio MA. Ciliary neurotrophic factor prevents acute lipid-induced insulin resistance by attenuating ceramide accumulation and phosphorylation of c-Jun N-terminal kinase in peripheral tissues. Endocrinology. 2006;147:2077–2085. doi: 10.1210/en.2005-1074. [DOI] [PubMed] [Google Scholar]
- 13.Borg ML, Andrews ZB, Duh EJ, Zechner R, Meikle PJ, Watt MJ. Pigment epithelium-derived factor regulates lipid metabolism via adipose triglyceride lipase. Diabetes. 2011;60:1458–1466. doi: 10.2337/db10-0845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rutti MF, Richard S, Penno A, von Eckardstein A, Hornemann T. An improved method to determine serine palmitoyltransferase activity. J Lipid Res. 2009;50:1237–1244. doi: 10.1194/jlr.D900001-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]