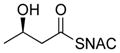
Table 1.
Summary of incorporation of partially assembled precursor analogues.
| Intermediate Analoguea,b | 13C Enrichment | Relative RAL yieldc | |
|---|---|---|---|
| 0 | none | none observed | 1.0 |
| 1 |
|
41% | 0.4 |
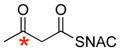
| 2 |
|
37% | 1.0 |
| 3 |
|
15% | 0.6 |
| 4 |
|
78% | 1.0 |
| 5 |
|
19% | 0.4 |
| 6 |
|
27% | 2.0 |
| 7 |
|
8% | 0.9 |
| 8 |
|
39% | 2.6 |
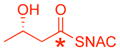
* indicates the location of 13C label;
ready precursor structures are shown in red;
resorcylic acid lactone (RAL) relative yield includes DHZ, and for unnatural precursor analogs, also the amount of corresponding DHZ analogs;
Compounds 9–12 have incorrect stereochemistry or functionality for conversion to DHZ and are therefore (except for 12) transformed to corresponding DHZ analogs;
production is the amount of DHZ analog formed compared to the total RAL production.