Abstract
In mammals, peripheral arterial chemoreceptors monitor blood chemicals (e.g. O2, CO2, H+, glucose) and maintain homeostasis via initiation of respiratory and cardiovascular reflexes. Whereas chemoreceptors in the carotid bodies (CBs), located bilaterally at the carotid bifurcation, control primarily respiratory functions, those in the more diffusely distributed aortic bodies (ABs) are thought to regulate mainly cardiovascular functions. Functionally, CBs sense partial pressure of O2 (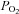 ), whereas ABs are considered sensors of O2 content. How these organs, with essentially a similar complement of chemoreceptor cells, differentially process these two different types of signals remains enigmatic. Here, we review evidence that implicates ATP as a central mediator during information processing in the CB. Recent data allow an integrative view concerning its interactions at purinergic P2X and P2Y receptors within the chemosensory complex that contains elements of a ‘quadripartite synapse’. We also discuss recent studies on the cellular physiology of ABs located near the aortic arch, as well as immunohistochemical evidence suggesting the presence of pathways for P2X receptor signalling. Finally, we present a hypothetical ‘quadripartite model’ to explain how ATP, released from red blood cells during hypoxia, could contribute to the ability of ABs to sense O2 content.
), whereas ABs are considered sensors of O2 content. How these organs, with essentially a similar complement of chemoreceptor cells, differentially process these two different types of signals remains enigmatic. Here, we review evidence that implicates ATP as a central mediator during information processing in the CB. Recent data allow an integrative view concerning its interactions at purinergic P2X and P2Y receptors within the chemosensory complex that contains elements of a ‘quadripartite synapse’. We also discuss recent studies on the cellular physiology of ABs located near the aortic arch, as well as immunohistochemical evidence suggesting the presence of pathways for P2X receptor signalling. Finally, we present a hypothetical ‘quadripartite model’ to explain how ATP, released from red blood cells during hypoxia, could contribute to the ability of ABs to sense O2 content.
 |
Nikol A. Piskuric (left) completed her PhD studies on the anatomical organization and cellular physiology of aortic versus carotid body chemoreceptors at McMaster University in 2012. She was the recipient of a Vanier Canada Graduate Scholarship from the Natural Sciences and Engineering Research Council of Canada during her doctoral studies. Colin A. Nurse obtained a PhD degree in Neurobiology from Harvard University in 1977. He has since held academic positions at McMaster University, where he is currently Professor of Biology, since 1994. His research interests are in the mechanisms of sensory processing at peripheral arterial chemoreceptors, and on the developmental regulation of O2 and CO2 chemosensitivity in adrenal chromaffin cells.
Introduction
Homeostasis in the mammalian respiratory and cardiovascular systems is maintained by reflexes that are initiated at specialized peripheral organs that sense changes in the chemical composition of arterial blood. These so-called peripheral arterial chemoreceptors are located strategically along the major arteries and contribute to the maintenance of O2 and CO2/H+ homeostasis (Gonzalez et al. 1994). One well-studied group is the bilateral pair of carotid bodies (CBs) located near the bifurcation of the common carotid artery. In response to reductions in the partial pressure of O2 (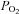 ), i.e. hypoxia, or increases in
), i.e. hypoxia, or increases in 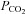 /H+ (acid hypercapnia), CB receptor cells depolarize and release excitatory neurotransmitters onto afferent terminals of the carotid sinus nerve (Gonzalez et al. 1994; Lopez-Barneo, 2003; Nurse, 2010). The resulting increase in sensory discharge is relayed to the central pattern generator in the brainstem, ultimately leading to hyperventilation and restoration of blood gas and pH homeostasis. It is now generally accepted that the CB is a polymodal sensor, capable of detecting other sensory modalities including low glucose and temperature (Lopez-Barneo, 2003; Kumar & Bin-Jaliah, 2007; Nurse, 2010). Additionally, CB chemoexcitation is also known to play a role in cardiovascular reflexes resulting in bradycardia and peripheral vasoconstriction mediated via changes in sympathetic and parasympathetic efferent activity (Alsberge et al. 1988; Kumar, 2009).
/H+ (acid hypercapnia), CB receptor cells depolarize and release excitatory neurotransmitters onto afferent terminals of the carotid sinus nerve (Gonzalez et al. 1994; Lopez-Barneo, 2003; Nurse, 2010). The resulting increase in sensory discharge is relayed to the central pattern generator in the brainstem, ultimately leading to hyperventilation and restoration of blood gas and pH homeostasis. It is now generally accepted that the CB is a polymodal sensor, capable of detecting other sensory modalities including low glucose and temperature (Lopez-Barneo, 2003; Kumar & Bin-Jaliah, 2007; Nurse, 2010). Additionally, CB chemoexcitation is also known to play a role in cardiovascular reflexes resulting in bradycardia and peripheral vasoconstriction mediated via changes in sympathetic and parasympathetic efferent activity (Alsberge et al. 1988; Kumar, 2009).
By contrast, another group of peripheral chemoreceptors known as the aortic bodies (ABs) has been poorly studied, and only recently has their cellular physiology been investigated (Piskuric & Nurse, 2012). These ABs are scattered diffusely along the thoracic and cervical vagus nerve and its branches, and are thought to act as ‘O2-content’ rather than ‘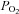 ’ sensors, eliciting primarily cardiovascular reflexes (Comroe, 1939; Lahiri et al. 1981). While there is a wealth of information on potential mechanisms by which the CB senses
’ sensors, eliciting primarily cardiovascular reflexes (Comroe, 1939; Lahiri et al. 1981). While there is a wealth of information on potential mechanisms by which the CB senses 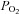 (Buckler, 2007; Peers et al. 2010), and the role of neurotransmitters in signal processing (Nurse, 2010; Nurse & Piskuric, 2012), virtually nothing is known about the mechanisms underlying the ability of ABs to monitor O2 content. This remains a major challenge, especially because the cellular components and morphology of the chemoreceptor complexes appear similar in both the CB and AB. Also, as illustrated in Fig. 1, the responses of their respective chemoreceptor cells appear similar (Piskuric & Nurse, 2012).
(Buckler, 2007; Peers et al. 2010), and the role of neurotransmitters in signal processing (Nurse, 2010; Nurse & Piskuric, 2012), virtually nothing is known about the mechanisms underlying the ability of ABs to monitor O2 content. This remains a major challenge, especially because the cellular components and morphology of the chemoreceptor complexes appear similar in both the CB and AB. Also, as illustrated in Fig. 1, the responses of their respective chemoreceptor cells appear similar (Piskuric & Nurse, 2012).
Figure 1. Intracellular Ca2+ responses in CB versus AB type I cells to various chemostimuli.
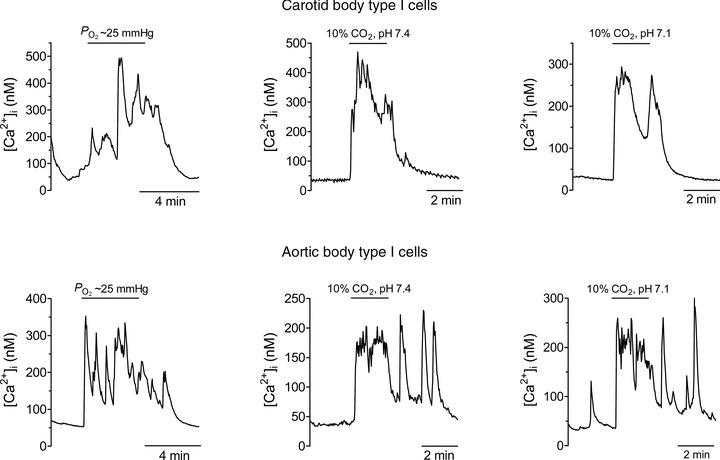
Note that type I cells from both organs respond similarly to a decrease in 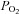 (hypoxia), isohydric hypercapnia and acid hypercapnia.
(hypoxia), isohydric hypercapnia and acid hypercapnia.
In this review, we examine the evidence for a central role of ATP and purinergic receptor signalling during information processing in the CB. Recent evidence suggesting contributions from glial cells is also included. Though the focus here is on ATP, it is recognized that many other neurochemicals, including dopamine, adenosine and ACh, play key roles in the physiological function of CBs. Space constraints preclude an adequate consideration of the relevant literature on those neurochemicals; however, the reader is referred to other reviews and articles on these topics (Eyzaguirre & Zapata, 1984; Shirahata et al. 2007; Iturriaga et al. 2009; Conde et al. 2012; Nurse & Piskuric, 2012). We also review recent data on the organization and cellular physiology of ABs, and present a novel hypothesis on how ATP release from red blood cells could contribute to their ability to monitor O2 content.
Anatomical organization of carotid bodies versus aortic bodies
Although more numerous, ABs are relatively small such that their combined total volume is less than that of one CB (McDonald & Blewett, 1981). In both cases, the chemosensory units comprise innervated chemoreceptor type I (glomus) cell clusters with interdigitating glia-like type II cells and their blood supply (McDonald & Mitchell, 1975; Hansen, 1981; Kummer & Neuhuber, 1989). The oval type I cells (∼10 μm diameter) possess catecholamine-containing dense-core and clear-core vesicles (Kummer & Neuhuber, 1989; Gonzalez et al. 1994). The glia-like (sustentacular) type II cells are ∼4-times fewer, and have elongated cell bodies with long processes that ensheath type I cells (McDonald, 1981; Kummer & Neuhuber, 1989). In the CB, nerve endings derive mainly from afferent neurones located in the petrosal ganglion (Gonzalez et al. 1994), though there is an efferent autonomic innervation as well (Wang et al. 1993; Campanucci et al. 2003). In the AB, innervation derives mainly from afferent nodose ganglion neurons, though in some cases local neurones may also contribute (Hansen, 1981; Kummer & Neuhuber, 1989; Piskuric et al. 2011). Both organs are penetrated by thin-walled fenestrated capillaries; however, whereas the CB receives an enormous blood flow from branches of multiple arterioles, one distant arteriole is thought to supply the comparatively low blood flow to the AB (McDonald & Blewett, 1981; McDonald & Larue, 1983).
ATP as an excitatory neurotransmitter between carotid body type I cells and petrosal afferent terminals
The excitatory effects of ATP on the CB chemosensory discharge have been known for many years, based on perfusion experiments (Jarisch et al. 1952; Spergel & Lahiri, 1993). In the rat CB, P2X receptor agonists evoked rapid cardiorespiratory reflexes suggesting the presence of endogenous purinoceptors (McQueen et al. 1998). In other studies in the isolated cat and rabbit petrosal ganglion, ATP caused a dose-dependent increase in sinus nerve discharge consistent with the presence of purinoceptors on chemoafferent neurones (Alcayaga et al. 2000; Soto et al. 2010). However, the advent of a functional co-culture model of rat CB type I cell clusters and petrosal neurones led to the demonstration that ATP was a key excitatory neurotransmitter during sensory transmission (Zhang et al. 2000; Nurse, 2010). In this model, the general P2 receptor blocker, suramin, partially inhibited both hypoxia- and hypercapnia-induced postsynaptic responses recorded in petrosal neurones, and both P2X2 and P2X3 purinergic subunits were immunolocalized to petrosal afferent terminals in the rat CB in situ (Prasad et al. 2001). Compelling evidence for a role of ATP and P2X receptors in CB chemosensory transmission was obtained following the use of a transgenic mouse model deficient in the P2X2 subunit (Rong et al. 2003). These mutant mice showed a marked attenuation of both the hypoxic ventilatory response and hypoxia-evoked afferent sinus nerve discharge. While the role of the P2X2 subunit was critical in this study, mice lacking the P2X3 subunit showed normal hypoxic ventilatory responses (Rong et al. 2003). However, more recent studies utilizing single fibre recordings have suggested a major role for P2X3-containing receptors in the sensory discharge in the rat CB (Niane et al. 2011). In general, the non-selective P2 blockers, i.e. suramin and PPADS, have been successfully used to inhibit hypoxia-evoked sinus nerve discharge in several species including rat, mouse and cat (Zhang et al. 2000; Rong et al. 2003; Iturriaga & Alcayaga, 2004; He et al. 2006; Zapata, 2007; Niane et al. 2011). Taken together these findings, along with the detection of hypoxia-evoked ATP release from whole CBs (Buttigieg & Nurse, 2004), support a major role for ATP and P2X2/3 receptors in CB sensory transmission. However, there is strong evidence that other excitatory co-transmitters (e.g. ACh, adenosine) are also involved (Zapata, 2007; Nurse, 2010; Conde et al. 2012).
Possible contribution of pannexin-1 channels in glia-like type II cells to chemotransmission via ATP-induced ATP release
The role of glia-like type II cells in CB function remained elusive for many years. These spindle-shaped cells ensheath type I cells and their close membrane appositions were once thought to include gap junctions (Kondo, 2002). A potential physiological role for type II cells in CB function was suggested by the observation that ATP induced a rise in intracellular Ca2+ in isolated rat type II cells via activation of G-protein-coupled P2Y2 receptors (Xu et al. 2003; Tse et al. 2012). Recent studies from our laboratory have suggested that activation of P2Y2 receptors by ATP or UTP on type II cells can lead to further release of ATP via opening of gap junction-like pannexin-1 channels, which were immunolocalized to type II cells (Zhang et al. 2012). These channels are now known to act as conduits for ATP release from a variety of cell types including glial cells (MacVicar & Thompson, 2010) and red blood cells (Locovei et al. 2006). In type II cells, both ATP and UTP activated a depolarizing inward current that was reversibly inhibited by low concentrations (5 μm) of carbenoxolone, a pannexin-1 channel blocker (Zhang et al. 2012). Moreover, selective activation of P2Y2 receptors on type II cells led in several cases to ATP release via pannexin-1 channels, detected using nearby petrosal neurones as biosensors (Zhang et al. 2012). These data suggest a novel role for type II cells in amplifying the ATP signal via the mechanism of ATP-induced ATP release.
Role of ATP in mediating efferent chemoreceptor inhibition via nitric oxide (NO)
An efferent inhibitory innervation of the CB, first described in the early 1970s, is now known to use NO as the mediator (reviewed in Campanucci & Nurse, 2007). This efferent innervation constitutes the fourth component of the proposed “quadripartite synapse”, which also contains the type I cells, type II cells, and afferent nerve terminals (Figure 2). Recent evidence suggests that ATP may also be involved in the activation of this NO signalling pathway, thereby contributing to a negative feedback regulation of its own release. The sources of NO are mainly axonal fibres derived from groups of neuronal nitric oxidesynthase (nNOS) -positive autonomic neurones embedded mainly in the glossopharyngeal (GPN) nerve (Campanucci et al. 2003). These GPN neurones and their processes express purinergic receptors containing P2X2, P2X3, P2X4 and P2X7 subunits, and their activation by ATP leads to increased excitability and a rise in intracellular calcium (Campanucci et al. 2006, 2012; Campanucci & Nurse, 2007). In a co-culture model of GPN neurones and CB type I clusters, stimulation of neuronal P2X receptors led to type I cell hyperpolarization that was prevented by NO scavengers (Campanucci et al. 2006). These data are consistent with a model whereby ATP released from type I cells during chemoexcitation could additionally excite GPN efferent fibres, leading to a rise in intracellular Ca2+ concentration, [Ca2+]i, activation of nNOS via Ca2+–calmodulin and local synthesis/release of NO near the chemoreceptor complex. The resulting NO-mediated hyperpolarization of type I cells could in turn limit or modulate the amount of ATP they release for a given chemosensory stimulus.
Figure 2. Model of the CB ‘quadripartite synapse’.
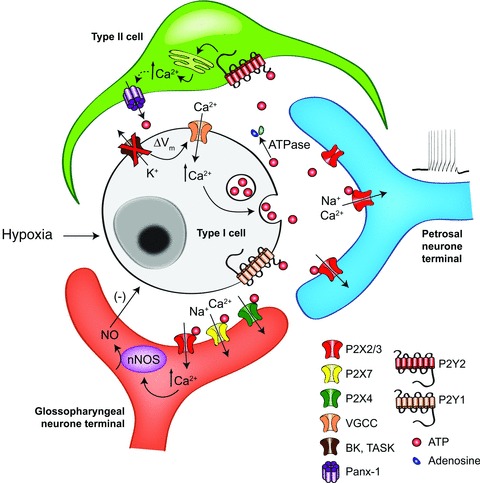
Release of ATP from type I cells during hypoxia excites sensory terminals; note role of type II cells in amplifying the ATP signal via P2Y2 receptors and the opening of pannexin-1 channels. Negative feedback pathways include inhibitory action of NO released from efferent nerves and ATP-mediated paracrine inhibition of type I cells. Note that the roles of other well-described CB neurotransmitters and neuromodulators, including ACh, dopamine and adenosine, as well as their respective signalling pathways (see Shirahata et al. 2007; Iturriaga et al. 2009; Nurse, 2010; Conde et al. 2012; Nurse & Piskuric, 2012), have been omitted here for clarity.
ATP as a negative feedback autocrine regulator of carotid body type I cell function via P2Y1 receptors
ATP may also regulate type I cell function via a negative feedback mechanism involving G-protein-coupled P2Y1 receptors (Xu et al. 2005). In the latter study, ATP (100 μm) strongly inhibited the hypoxia-induced elevation in [Ca2+]i in type I cells. The mechanism(s) involved closure of an unidentified resting conductance(s), causing membrane hyperpolarization that antagonized the hypoxia-induced depolarizing receptor potential (Xu et al. 2005). This negative feedback pathway provides an alternative route by which ATP can regulate its own levels at the synapse, and therefore the degree of afferent nerve excitation during chemotransduction (Xu et al. 2005; Tse et al. 2012). However, this effect is counteracted by the positive feedback action of adenosine, a breakdown product of ATP. On the presynaptic side, adenosine may enhance membrane depolarization and intracellular Ca2+ responses in type I cells via autocrine–paracrine activation of A2a receptors (Xu et al. 2006; Fitzgerald et al. 2009; Nurse & Piskuric, 2012; Tse et al. 2012). On the postsynaptic side, adenosine may also augment sinus nerve discharge via A2a receptors on afferent nerve terminals (Conde et al. 2012).
ATP as a putative excitatory neurotransmitter at aortic body type I cells
At the vagus-recurrent laryngeal nerve bifurcation, tyrosine hydroxylase (TH)-immunopositive AB type I cells are contacted by P2X2- and P2X3-immunopositive nerve terminals (Piskuric et al. 2011). These terminals probably derive from P2X2- and P2X3-expressing nodose ganglion neurones (Burnstock, 2009; Song et al. 2012), since this sensory ganglion is thought to be the major source of AB afferent innervation (Hansen, 1981; Kummer & Neuhuber, 1989). Interestingly, ∼25% of local neurones are also P2X2- and/or P2X3-immunoreactive and at least some of them appear to innervate type I cells (Piskuric et al. 2011). Subsets of these local neurones were recently found to be excited by exogenous ATP acting via P2X2/3 receptors (authors’ unpublished observations). As AB local neurones respond to several chemostimuli (e.g. hypoxia) via increases in cytosolic Ca2+ (Piskuric & Nurse, 2012), it is possible that at least some of them are sensory, analogous to CB petrosal chemoafferent neurones.
Does ATP release from red blood cells via pannexin-1 channels contribute to the ability of the aortic body to sense O2 content?
From the time ABs were first considered ‘O2-content’ sensors, a role for red blood cells (RBCs) was implicated (Lahiri et al. 1981). Because of their low blood flow, ABs were thought to depend on O2 carried by haemoglobin (Hb) to satisfy their energy demands (Lahiri et al. 1981). In view of the emerging role of the RBCs as O2 sensors, and the evidence that hypoxia evokes ATP release from RBCs (Ellsworth et al. 1995), it may well be that these cells play a direct role in AB chemosensing. Interestingly, the trigger for ATP release from RBCs is a decrease in O2 content as sensed by a change in Hb conformation upon desaturation (Ellsworth et al. 2009). Furthermore, the same channels that appear to mediate ATP-induced ATP release from CB type II cells, i.e. pannexin-1 channels (Zhang et al. 2012), have been recently shown to mediate hypoxia-evoked ATP release from RBCs (Sridharan et al. 2010). Thus, the RBC, acting as a ‘mobile O2-content sensor’ may initiate a systemic reflex in response to local tissue hypoxia, via ATP release. Given the high permeability of AB blood vessels (McDonald & Blewett, 1981), it is possible that this ATP could extravasate into the interstitial fluid and activate P2X2/3 receptors on local neurones or nerve terminals apposed to type I cells (Fig. 3). This effect of hypoxia on RBCs would facilitate that on AB type I cells, rendering the RBC equivalent to an ‘ATP amplifier’ at the AB chemosensory synapse.
Figure 3. Model of the AB ‘quadripartite complex’.
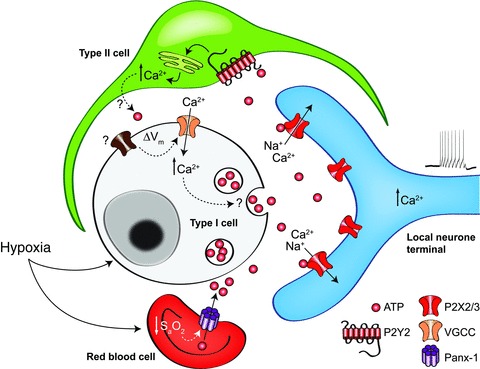
This hypothetical model is based on the AB at the left vagus-recurrent laryngeal nerve bifurcation. Note the presence of P2X-expressing local neurones and nerve terminals apposed to type I cells, as well as the proposed role of red blood cells as AB ‘O2 content’ sensors.
Closing remarks
Compelling evidence has been obtained over the last ∼12 years supporting a major role for ATP in signal processing in the CB. Though speculative, this review has examined more recent circumstantial evidence for ATP as an important signal in the AB as well. In the case of the CB, we propose an expanded role for ATP given the recent demonstration that glial-like type II cells could amplify the ATP signal via the mechanism of ATP-induced ATP release (Zhang et al. 2012). These new developments suggest that the CB contains the elements of a ‘quadripartite synapse’ (Fig. 2). In this model, the initial vesicular release of ATP from type I cells during chemotransduction could lead to paracrine activation of P2Y2 receptors on contiguous type II cells; the resulting opening of pannexin-1 channels could provide an auxiliary pathway for non-vesicular ATP release from type II cells. The total extracellular ATP pool, in combination with the pre- and post-synaptic actions of its breakdown product adenosine, appears to provide the major excitatory drive to the CB afferent nerve terminals (Nurse, 2010; Conde et al. 2012; Tse et al. 2012).
Besides the presence of nucleotidases that degrade ATP, a number of autocrine–paracrine, negative feedback pathways may help control ATP levels and therefore regulate afferent firing. These include: (i) ATP-mediated hyperpolarization of type I cells via G-protein-coupled P2Y1 receptors (Xu et al. 2005; Tse et al. 2012); (ii) ATP-mediated excitation of autonomic efferent terminals via P2X receptors, leading to synthesis/release of NO, which in turn causes type I cell hyperpolarization (Campanucci et al. 2006, 2012; Campanucci & Nurse, 2007); and (iii) closure of pannexin-1 channels in type II cells by high extracellular ATP concentrations (Dubyak, 2009).
In the case of the AB, it appears that the basic chemosensing mechanisms at the level of the type I cells may not be fundamentally different from those in the CB. Though further electrophysiological studies are required to validate this point, their intracellular Ca2+ responses to changes in 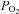 (and
(and 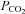 /pH) appear similar (Fig. 1; see also Piskuric & Nurse, 2012). If true, how does the AB sense blood O2 content in contrast to the CB which senses
/pH) appear similar (Fig. 1; see also Piskuric & Nurse, 2012). If true, how does the AB sense blood O2 content in contrast to the CB which senses 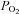 ? To facilitate future investigations, we propose a ‘quadripartite model’ where AB type I cells, type II cells, local ‘sensory’ neurones and/or afferent nerve endings, and RBCs are the major components (Fig. 3). The model relies on previous work demonstrating that hypoxaemia stimulates ATP release from RBCs via pannexin-1 channels (Ellsworth et al. 2009; Sridharan et al. 2010). This RBC-derived ATP is proposed to extravasate via fenestrated capillaries and contribute to excitation of sensory afferent terminals and/or local neurones. The model provides a plausible mechanism by which ABs can sense blood O2 content, and the pathway is facilitated by the relatively low blood flow to the organ. By contrast, the large blood flow to the CB would render the contribution of any RBC-derived ATP negligible. Tests of these models and their predictions remain a formidable challenge for future investigations into the contrasting physiology of the CB and AB.
? To facilitate future investigations, we propose a ‘quadripartite model’ where AB type I cells, type II cells, local ‘sensory’ neurones and/or afferent nerve endings, and RBCs are the major components (Fig. 3). The model relies on previous work demonstrating that hypoxaemia stimulates ATP release from RBCs via pannexin-1 channels (Ellsworth et al. 2009; Sridharan et al. 2010). This RBC-derived ATP is proposed to extravasate via fenestrated capillaries and contribute to excitation of sensory afferent terminals and/or local neurones. The model provides a plausible mechanism by which ABs can sense blood O2 content, and the pathway is facilitated by the relatively low blood flow to the organ. By contrast, the large blood flow to the CB would render the contribution of any RBC-derived ATP negligible. Tests of these models and their predictions remain a formidable challenge for future investigations into the contrasting physiology of the CB and AB.
Acknowledgments
We acknowledge the key contributions of several colleagues to the ideas expressed in this review, especially Min Zhang and Cathy Vollmer. Work in the authors’ laboratory was supported by grants from the Canadian Institutes of Health Research (CIHR) to C.A.N. In addition N.A.P. was supported by a Vanier Canada Graduate Scholarship from the Natural Sciences and Engineering Research Council of Canada (NSERC).
Glossary
- AB
aortic body
- CB
carotid body
- GPN
glossopharyngeal
- RBC
red blood cell
References
- Alcayaga J, Cerpa V, Retamal M, Arroyo J, Iturriaga R, Zapata P. Adenosine triphosphate-induced peripheral nerve discharges generated from the cat petrosal ganglion in vitro. Neurosci Lett. 2000;282:185–188. doi: 10.1016/s0304-3940(00)00896-x. [DOI] [PubMed] [Google Scholar]
- Alsberge M, Magno M, Lipschutz M. Carotid body control of bronchial circulation in sheep. J Appl Physiol. 1988;65:1152–1156. doi: 10.1152/jappl.1988.65.3.1152. [DOI] [PubMed] [Google Scholar]
- Buckler KJ. TASK-like potassium channels and oxygen sensing in the carotid body. Respir Physiol Neurobiol. 2007;157:55–64. doi: 10.1016/j.resp.2007.02.013. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Purines and sensory nerves. Handb Exp Pharmacol. 2009;194:333–392. doi: 10.1007/978-3-540-79090-7_10. [DOI] [PubMed] [Google Scholar]
- Buttigieg J, Nurse CA. Detection of hypoxia-evoked ATP release from chemoreceptor cells of the rat carotid body. Biochem Biophys Res Commun. 2004;322:82–87. doi: 10.1016/j.bbrc.2004.07.081. [DOI] [PubMed] [Google Scholar]
- Campanucci VA, Dookhoo L, Vollmer C, Nurse CA. Modulation of the carotid body sensory discharge by NO: an up-dated hypothesis. Respir Physiol Neurobiol. 2012;184:149–157. doi: 10.1016/j.resp.2012.04.005. [DOI] [PubMed] [Google Scholar]
- Campanucci VA, Fearon IM, Nurse CA. A novel O2-sensing mechanism in rat glossopharyngeal neurones mediated by a halothane-inhibitable background K+ conductance. J Physiol. 2003;548:731–743. doi: 10.1113/jphysiol.2002.035998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campanucci VA, Nurse CA. Autonomic innervation of the carotid body: role in efferent inhibition. Respir Physiol Neurobiol. 2007;157:83–92. doi: 10.1016/j.resp.2007.01.020. [DOI] [PubMed] [Google Scholar]
- Campanucci VA, Zhang M, Vollmer C, Nurse CA. Expression of multiple P2X receptors by glossopharyngeal neurons projecting to rat carotid body O2-chemoreceptors: role in nitric oxide-mediated efferent inhibition. J Neurosci. 2006;26:9482–9493. doi: 10.1523/JNEUROSCI.1672-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comroe JHJ. The location and function of the chemoreceptors of the aorta. Am J Physiol. 1939;127:176–191. [Google Scholar]
- Conde SV, Monteiro EC, Rigual R, Obeso A, Gonzalez C. Hypoxic intensity: a determinant for the contribution of ATP and adenosine to the genesis of carotid body chemosensory activity. J Appl Physiol. 2012;112:2002–2010. doi: 10.1152/japplphysiol.01617.2011. [DOI] [PubMed] [Google Scholar]
- Dubyak GR. Both sides now: multiple interactions of ATP with pannexin-1 hemichannels. Focus on ‘A permeant regulating its permeation pore: inhibition of pannexin 1 channels by ATP’. Am J Physiol Cell Physiol. 2009;296:C235–C241. doi: 10.1152/ajpcell.00639.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellsworth ML, Ellis CG, Goldman D, Stephenson AH, Dietrich HH, Sprague RS. Erythrocytes: oxygen sensors and modulators of vascular tone. Physiology (Bethesda) 2009;24:107–116. doi: 10.1152/physiol.00038.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellsworth ML, Forrester T, Ellis CG, Dietrich HH. The erythrocyte as a regulator of vascular tone. Am J Physiol Heart Circ Physiol. 1995;269:H2155–H2161. doi: 10.1152/ajpheart.1995.269.6.H2155. [DOI] [PubMed] [Google Scholar]
- Eyzaguirre C, Zapata P. Perspectives in carotid body research. J Appl Physiol. 1984;57:931–957. doi: 10.1152/jappl.1984.57.4.931. [DOI] [PubMed] [Google Scholar]
- Fitzgerald RS, Shirahata M, Chang I. The impact of adenosine and an A2A adenosine receptor agonist on the ACh-induced increase in intracellular calcium of the glomus cells of the cat carotid body. Brain Res. 2009;1301:20–33. doi: 10.1016/j.brainres.2009.08.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez C, Almaraz L, Obeso A, Rigual R. Carotid body chemoreceptors: from natural stimuli to sensory discharges. Physiol Rev. 1994;74:829–898. doi: 10.1152/physrev.1994.74.4.829. [DOI] [PubMed] [Google Scholar]
- Hansen JT. Innervation of the rat aortic (subclavian) body: an ultrastructural study following axonal degeneration. J Ultrastruct Res. 1981;74:83–94. doi: 10.1016/s0022-5320(81)80111-6. [DOI] [PubMed] [Google Scholar]
- He L, Chen J, Dinger B, Stensaas L, Fidone S. Effect of chronic hypoxia on purinergic synaptic transmission in rat carotid body. J Appl Physiol. 2006;100:157–162. doi: 10.1152/japplphysiol.00859.2005. [DOI] [PubMed] [Google Scholar]
- Iturriaga R, Alcayaga J. Neurotransmission in the carotid body: transmitters and modulators between glomus cells and petrosal ganglion nerve terminals. Brain Res Brain Res Rev. 2004;47:46–53. doi: 10.1016/j.brainresrev.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Iturriaga R, Alcayaga J, Gonzalez C. Neurotransmitters in carotid body function: the case of dopamine – invited article. Adv Exp Med Biol. 2009;648:137–143. doi: 10.1007/978-90-481-2259-2_16. [DOI] [PubMed] [Google Scholar]
- Jarisch A, Landgren S, Neil E, Zotterman Y. Impulse activity in the carotid sinus nerve following intra-carotid injection of potassium chloride, veratrine, sodium citrate, adenosine-triphosphate and alpha-dinitrophenol. Acta Physiol Scand. 1952;25:195–211. doi: 10.1111/j.1748-1716.1952.tb00872.x. [DOI] [PubMed] [Google Scholar]
- Kondo H. Are there gap junctions between chief (glomus, type I) cells in the carotid body chemoreceptor? A review. Microsc Res Tech. 2002;59:227–233. doi: 10.1002/jemt.10196. [DOI] [PubMed] [Google Scholar]
- Kumar P. Systemic effects resulting from carotid body stimulation-invited article. Adv Exp Med Biol. 2009;648:223–233. doi: 10.1007/978-90-481-2259-2_26. [DOI] [PubMed] [Google Scholar]
- Kumar P, Bin-Jaliah I. Adequate stimuli of the carotid body: more than an oxygen sensor? Respir Physiol Neurobiol. 2007;157:12–21. doi: 10.1016/j.resp.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Kummer W, Neuhuber WL. Vagal paraganglia of the rat. J Electron Microsc Tech. 1989;12:343–355. doi: 10.1002/jemt.1060120407. [DOI] [PubMed] [Google Scholar]
- Lahiri S, Mulligan E, Nishino T, Mokashi A, Davies RO. Relative responses of aortic body and carotid body chemoreceptors to carboxyhemoglobinemia. J Appl Physiol. 1981;50:580–586. doi: 10.1152/jappl.1981.50.3.580. [DOI] [PubMed] [Google Scholar]
- Locovei S, Bao L, Dahl G. Pannexin 1 in erythrocytes: function without a gap. Proc Natl Acad Sci U S A. 2006;103:7655–7659. doi: 10.1073/pnas.0601037103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Barneo J. Oxygen and glucose sensing by carotid body glomus cells. Curr Opin Neurobiol. 2003;13:493–499. doi: 10.1016/s0959-4388(03)00093-x. [DOI] [PubMed] [Google Scholar]
- McDonald DM. Peripheral chemoreceptors: structure–function relationships of the carotid body. In: Hornbein TF, editor. Regulation of Breathing. Lung Biology in Health and Disease. Vol. 17. New York: Marcel Dekker; 1981. pp. 105–320. [Google Scholar]
- McDonald DM, Blewett RW. Location and size of carotid body-like organs (paraganglia) revealed in rats by the permeability of blood vessels to Evans blue dye. J Neurocytol. 1981;10:607–643. doi: 10.1007/BF01262593. [DOI] [PubMed] [Google Scholar]
- McDonald DM, Larue DT. The ultrastructure and connections of blood vessels supplying the rat carotid body and carotid sinus. J Neurocytol. 1983;12:117–153. doi: 10.1007/BF01148090. [DOI] [PubMed] [Google Scholar]
- McDonald DM, Mitchell RA. The innervation of glomus cells, ganglion cells and blood vessels inthe rat carotid body: a quantitative ultrastructural analysis. J Neurocytol. 1975;4:177–230. [Google Scholar]
- McQueen DS, Bond SM, Moores C, Chessell I, Humphrey PP, Dowd E. Activation of P2X receptors for adenosine triphosphate evokes cardiorespiratory reflexes in anaesthetized rats. J Physiol. 1998;507:843–855. doi: 10.1111/j.1469-7793.1998.843bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacVicar BA, Thompson RJ. Non-junction functions of pannexin-1 channels. Trends Neurosci. 2010;33:93–102. doi: 10.1016/j.tins.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Niane LM, Donnelly DF, Joseph V, Bairam A. Ventilatory and carotid body chemoreceptor responses to purinergic P2X receptor antagonists in newborn rats. J Appl Physiol. 2011;110:83–94. doi: 10.1152/japplphysiol.00871.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurse CA. Neurotransmitter and neuromodulatory mechanisms at peripheral arterial chemoreceptors. Exp Physiol. 2010;95:657–667. doi: 10.1113/expphysiol.2009.049312. [DOI] [PubMed] [Google Scholar]
- Nurse CA, Piskuric NA. Signal processing at mammalian carotid body chemoreceptors. Semin Cell Dev Biol. 2012 doi: 10.1016/j.semcdb.2012.09.006. (Epublish ahead of print) [DOI] [PubMed] [Google Scholar]
- Peers C, Wyatt CN, Evans AM. Mechanisms for acute oxygen sensing in the carotid body. Respir Physiol Neurobiol. 2010;174:292–298. doi: 10.1016/j.resp.2010.08.010. [DOI] [PubMed] [Google Scholar]
- Piskuric NA, Nurse CA. Effects of chemostimuli on [Ca2+]i responses of rat aortic body type I cells and endogenous local neurons: comparison with carotid body cells. J Physiol. 2012;590:2121–2135. doi: 10.1113/jphysiol.2012.229468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piskuric NA, Vollmer C, Nurse CA. Confocal immunofluorescence study of rat aortic body chemoreceptors and associated neurons in situ and in vitro. J Comp Neurol. 2011;519:856–873. doi: 10.1002/cne.22553. [DOI] [PubMed] [Google Scholar]
- Prasad M, Fearon IM, Zhang M, Laing M, Vollmer C, Nurse CA. Expression of P2X2 and P2X3 receptor subunits in rat carotid body afferent neurones: role in chemosensory signalling. J Physiol. 2001;537:667–677. doi: 10.1111/j.1469-7793.2001.00667.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong W, Gourine AV, Cockayne DA, Xiang Z, Ford AP, Spyer KM, Burnstock G. Pivotal role of nucleotide P2X2 receptor subunit of the ATP-gated ion channel mediating ventilatory responses to hypoxia. J Neurosci. 2003;23:11315–11321. doi: 10.1523/JNEUROSCI.23-36-11315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirahata M, Balbir A, Otsubo T, Fitzgerald RS. Role of acetylcholine in neurotransmission of the carotid body. Respir Physiol Neurobiol. 2007;157:93–105. doi: 10.1016/j.resp.2006.12.010. [DOI] [PubMed] [Google Scholar]
- Song X, Gao X, Guo D, Yu Q, Guo W, He C, Burnstock G, Xiang Z. Expression of P2X2 and P2X3 receptors in the rat carotid sinus, aortic arch, vena cava, and heart, as well as petrosal and nodose ganglia. Purinergic Signal. 2012;8:15–22. doi: 10.1007/s11302-011-9249-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto CR, Ortiz FC, Vargas RV, Arroyo J, Alcayaga J. Responses induced by acetylcholine and ATP in the rabbit petrosal ganglion. Respir Physiol Neurobiol. 2010;172:114–121. doi: 10.1016/j.resp.2010.05.003. [DOI] [PubMed] [Google Scholar]
- Spergel D, Lahiri S. Differential modulation by extracellular ATP of carotid chemosensory responses. J Appl Physiol. 1993;74:3052–3056. doi: 10.1152/jappl.1993.74.6.3052. [DOI] [PubMed] [Google Scholar]
- Sridharan M, Adderley SP, Bowles EA, Egan TM, Stephenson AH, Ellsworth ML, Sprague RS. Pannexin 1 is the conduit for low oxygen tension-induced ATP release from human erythrocytes. Am J Physiol Heart Circ Physiol. 2010;299:H1146–H1152. doi: 10.1152/ajpheart.00301.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse A, Yan L, Lee AK, Tse FW. Autocrine and paracrine actions of ATP in rat carotid body. Can J Physiol Pharmacol. 2012;90:705–711. doi: 10.1139/y2012-054. [DOI] [PubMed] [Google Scholar]
- Wang ZZ, Bredt DS, Fidone SJ, Stensaas LJ. Neurons synthesizing nitric oxide innervate the mammalian carotid body. J Comp Neurol. 1993;336:419–432. doi: 10.1002/cne.903360308. [DOI] [PubMed] [Google Scholar]
- Xu F, Xu J, Tse FW, Tse A. Adenosine stimulates depolarization and rise in cytoplasmic [Ca2+] in type I cells of rat carotid bodies. Am J Physiol Cell Physiol. 2006;290:C1592–C1598. doi: 10.1152/ajpcell.00546.2005. [DOI] [PubMed] [Google Scholar]
- Xu J, Tse FW, Tse A. ATP triggers intracellular Ca2+ release in type II cells of the rat carotid body. J Physiol. 2003;549:739–747. doi: 10.1113/jphysiol.2003.039735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Xu F, Tse FW, Tse A. ATP inhibits the hypoxia response in type I cells of rat carotid bodies. J Neurochem. 2005;92:1419–1430. doi: 10.1111/j.1471-4159.2004.02978.x. [DOI] [PubMed] [Google Scholar]
- Zapata P. Is ATP a suitable co-transmitter in carotid body arterial chemoreceptors? Respir Physiol Neurobiol. 2007;157:106–115. doi: 10.1016/j.resp.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Zhang M, Piskuric NA, Vollmer C, Nurse CA. P2Y2 receptor activation opens pannexin-1 channels in rat carotid body type II cells: potential role in amplifying the neurotransmitter ATP. J Physiol. 2012;590:4335–4350. doi: 10.1113/jphysiol.2012.236265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Zhong H, Vollmer C, Nurse CA. Co-release of ATP and ACh mediates hypoxic signalling at rat carotid body chemoreceptors. J Physiol. 2000;525:143–158. doi: 10.1111/j.1469-7793.2000.t01-1-00143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


