Figure 3. Model showing the interactive effects of chronically excessive fructose intake among multiple organ systems.
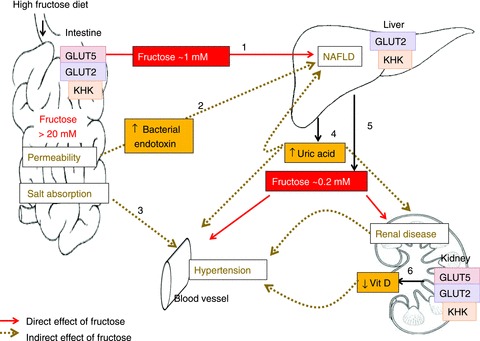
In rodent models, consumption of high-fructose diets may lead to >20 mm free fructose in the intestinal lumen and, after transport by GLUTs 5 and 2 and metabolism by KHK, yields >1 mm fructose concentration in the portal vein (step 1). The liver catabolizes most of the fructose, but under chronic conditions, may increase lipogenesis in this organ by the unregulated production of two carbon precursors, eventually leading to NAFLD. Step 2, high luminal fructose may also increase transepithelial permeability, allowing the passage of bacterial endotoxins that induce inflammatory reactions in hepatocytes, contributing to NAFLD. Step3, GLUT5 may interact with intestinal chloride transporters as to perturb electrolyte homeostasis and contribute to hypertension. Step 4, the chronic delivery of high fructose in the portal blood lowers ATP levels in hepatocytes and increases uric acid production which may contribute to the etiology not only of NAFLD but also of hypertension and renal disease. Step 5, as the liver removes most of the fructose, peripheral blood level is only ∼0.2 mm. Thus, with the exception of the liver and intestine, organ systems thought to be negatively impacted by a high fructose intake actually are bathed in modest fructose concentrations < 1 mM during postprandial periods, and < 0.2 mM between meals. Step 6: however, a chronic, ∼10-fold increase in these modest (relative to glucose) peripheral fructose concentrations seems sufficient to cause marked increases in proximal tubular cells, GLUT5 and GLUT2 expression, and renal hypertrophy, negatively impacting the synthesis of 1,25 (OH)2D3, contributing potentially to hypertension.
