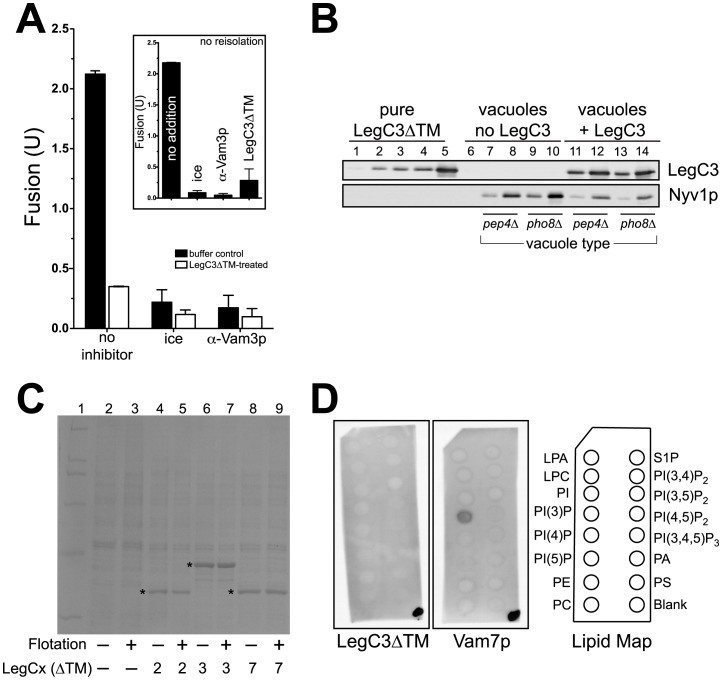
Figure 3. LegC3 associates with the vacuole in the absence of a transmembrane domain, inhibiting membrane fusion.
(A) 300 µl reactions containing 200 µg vacuoles purified from either BJ3505 or DKY6281 were incubated at 27°C for 15 min, in either the absence or presence of 3.5 µM LegC3ΔTM protein. All incubations were performed in PS buffer (20 mM Pipes-KOH pH 6.8, 200 mM sorbitol). After incubation, vacuole mixtures were mixed with a 15% (w/v) Ficoll solution in PS buffer to obtain a final concentration of 8% (w/v) Ficoll, then added to a 11×34 mm Thickwall polyallomer tube (Beckman). Discontinuous steps consisting of 8%, 4%, and 0% Ficoll (PS buffer) layers were overlayed to fill the remaining volume in each tube. Vacuoles were reisolated via flotation in a TLS-55 swinging bucket rotor (Beckman, 134,000×g, 30 min, 4°C). Standard fusion reactions were performed from matching pairs of vacuoles that either had not been incubated (black bars), or had been incubated (white bars) with LegC3ΔTM. Due to day-to-day variability, fusion for each reisolated pair was normalized to the mean of the “no inhibitor” reactions (no LegC3∶2.11±1.06 U, n = 4;+LegC3∶0.35±0.11 U, n = 4); error is standard deviation from mean. Standard low-salt fusion reactions were performed from these same vacuoles without reisolation, to show LegC3ΔTM inhibition (inset). These reactions were normalized to the mean of the “no addition” reactions (2.18±0.71 U, n = 3); error is standard deviation from mean. (B) 0.25 µg and 1.0 µg of each refloated vacuole type were separated on SDS-PAGE, along with a standard curve (10, 50, 100, 200, 500 ng) of purified LegC3ΔTM protein. LegC3ΔTM and the vacuole-bound SNARE Nyv1p were detected with standard immunoblotting techniques. Estimation of LegC3ΔTM protein on vacuoles was carried out via software-based densitometry (ImageJ v. 1.43u, http://rsb.info.nih.gov/ij). (C) 100 µg yeast vacuoles isolated from BJ3505 were incubated (15 min, 27°C) without, or with 3.5 µM purified LegC2ΔTM, LegC3ΔTM, or LegC7ΔTM, in PS buffer, to a final volume of 300 µl. Samples were refloated as in (A), and equal fractions of vacuolar material prior to, and after, reisolation were separated via SDS-PAGE, and visualized with Coomassie staining. Purified Leg proteins are denoted with an asterisk. (D) Individual PIP Strip™ membranes (Echelon Bioscience) were used via suggested Manufacturer’s protocol, and incubated with 0.5 µg/ml purified LegC3ΔTM (right) or Vam7p (left). After washing, proteins were detected via standard immunoblotting techniques. Prior to blocking the membrane, 0.5 µl of the appropriate protein solution was applied to the lower-right corner of the membrane, to confirm antibody activity. Lipid abbreviations: LPA, lysophosphatidic acid; LPC, lysophosphocholine; PI, phosphatidylinositol; PI(3)P, PI 3-phosphate; PI(4)P, PI 4-phosphate; PI(5)P, PI 5-phosphate; PE, phosphatidylethanolamine; PC, phosphatidylcholine; S1P, sphingosine-1-phosphate; PI(3,4)P2, PI 3,4-bisphosphate; PI(3,5)P2, PI 3,5-bisphosphate; PI(4,5)P2, PI 4,5 bisphosphate; PI(3,4,5)P3, PI 3,4,5-trisphosphate; PA, phosphatidic acid; PS, phosphatidylserine.