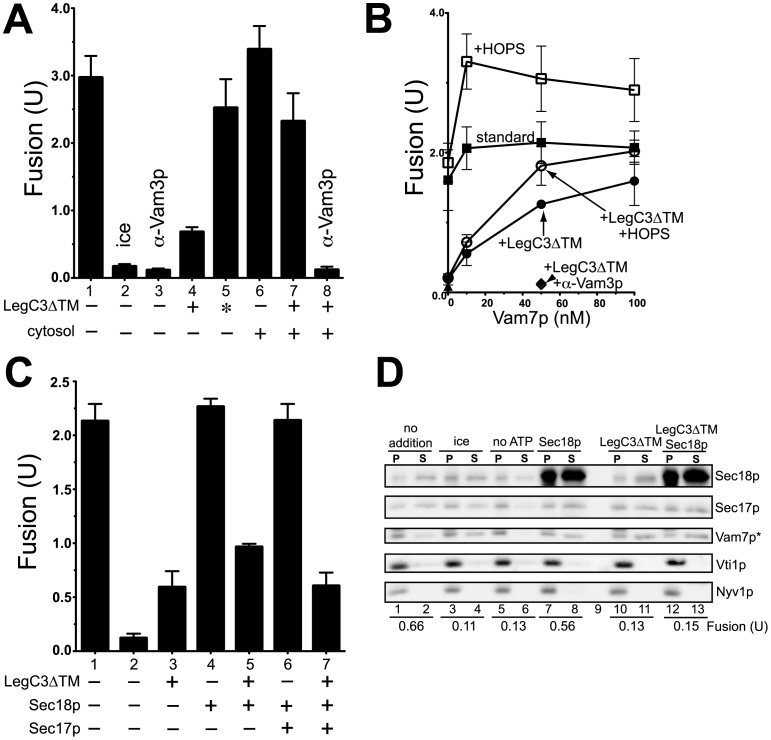
Figure 4. Yeast cytosol and a soluble vacuolar SNARE bypass LegC3-mediated fusion inhibition, but not SNARE chaperones.
(A) Low-salt vacuole fusion reactions were incubated with either LegC3ΔTM protein (3.5 µM), heat-inactivated LegC3ΔTM (asterisk, 98°C, 15 min), yeast cytosol (31 µg), or α-Vam3p. All reactions lacking LegC3ΔTM contained no-protein buffer. Yeast cytosol was prepared from a phosphatase-deficient yeast strain, as in [93]. (B) Low-salt fusion reactions containing either 3.5 µM LegC3ΔTM (circles) or no-protein buffer (squared) were incubated with increasing concentrations of purified Vam7p SNARE protein; with (open symbols) or without (closed symbols) 20 nM purified HOPS complex. (C) Low-salt fusion reactions contained either LegC3ΔTM (3.5 µM), Sec17p (80 nM), or Sec18p (80 nM). Reactions lacking LegC3ΔTM contained the appropriate buffer control. All fusion reactions in (A–C) were performed in triplicate; error is standard deviation. (D) 3×volume (90 µl) low-salt fusion reactions were incubated at either 0°C or 27°C for 30 min with LegC3ΔTM buffer control, 80 nM Sec18p, 3.5 µM LegC3ΔTM, or without ATP. After incubation, reactions were placed on ice for 5 min. One 30-µl aliquot from each reaction was used to assay fusion standard procedures, and one 30 µl aliquot from each reaction was diluted with 30 µl ice-cold PS buffer, and centrifuged to pellet membranes (6200×g, 6 min, 4°C). Supernatants were harvested, and equal portions of each supernatant (S) and vacuole pellet (P) were separated via SDS-PAGE. Proteins were detected by immunoblotting; Vam7p detection required a longer exposure time than the other listed proteins (asterisk). Blot and fusion values corresponding to this blot are representative of three independent experiments.