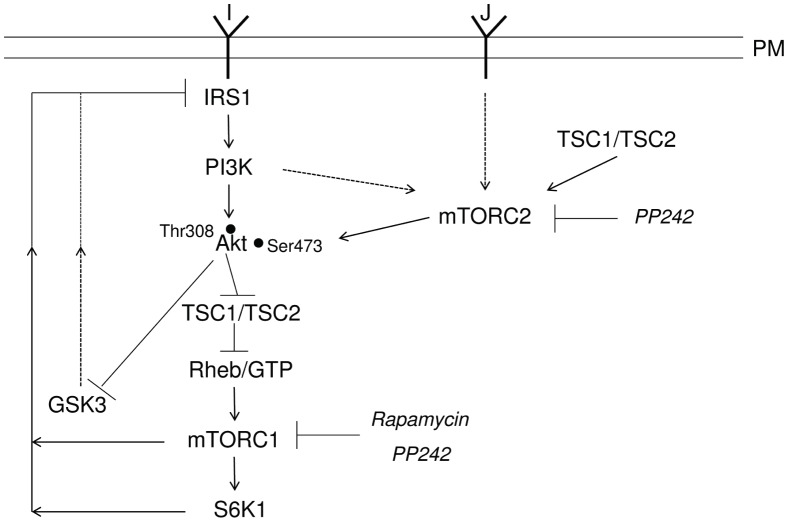
Figure 6. Interaction between IRS1−PI3K−Akt signaling pathway and mTOR.
Upon insulin binding insulin receptor activates, through insulin receptor substrate 1 (IRS1), phosphatidylinositol-3-kinase (PI3K), which results in Akt phosphorylation at 308Thr residue via Phosphatidylinositol 4,5-bisphosphate (PIP2) to Phosphatidylinositol 3,4,5-bisphosphate (PIP3) conversion followed by Akt and PDK1 recruitment to plasma membrane (not shown). A factor J present in duodenal-jejunal conditioned medium activates, possibly via the tuberous sclerosis complex TSC1-TSC2, the mammlian target of rapamycin complex 2 (mTORC2). mTORC2 also appears to be regulated by the PI3K pathway and phosphorylates Akt at 473Ser residue. Through TSC1-TSC2 and the GTPase Rheb, Akt activates mTORC1 and its direct substrate S6K1. Akt also inhibits GSK3. S6K1, mTORC1 and GSK3 phosphorylate serine residues on IRS1, thus attenuating insulin signalling. Hypothetical signalling is denoted by dotted lines.