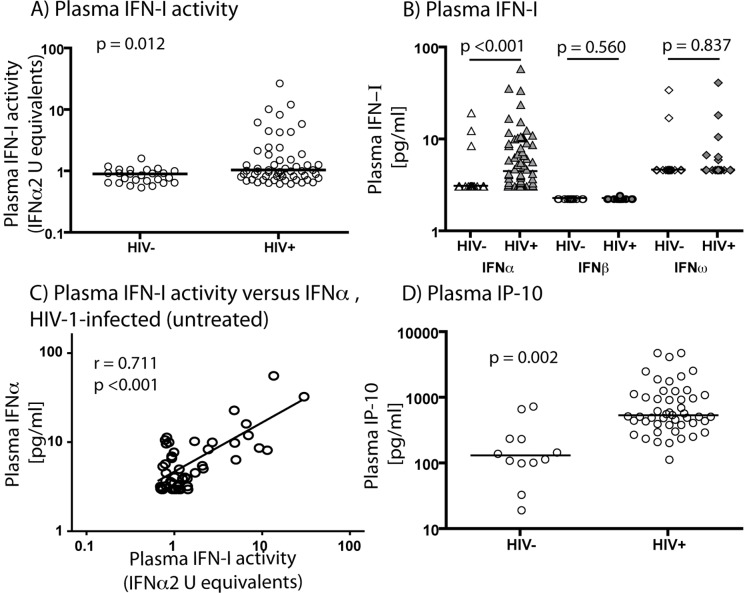
Figure 1. IFNα and its signature are increased in plasma of HIV-1-infected subjects not receiving ART.
Plasma IFN-I bioactivity measured by the iLite™ bioassay was increased in plasma of HIV-1-infected subjects in comparison to uninfected subjects (median 1.04 IFNα2 equivalent units for HIV-1-infected subjects, median 0.89 IFNα2 equivalent units for uninfected subjects, p = 0.012) (A). IFNα was increased in plasma of HIV-1-infected subjects in comparison to uninfected subjects (median 4.27 pg/ml for HIV-1-infected subjects, median 3.13 pg/ml for uninfected subjects, p<0.001) (B). IFNβ was not increased in plasma of HIV-1-infected subjects compared to uninfected subjects (median 2.34 pg/ml for HIV-1-infected subjects, median 2.34 pg/ml for uninfected subjects, p = 0.560) (B). IFNω was not increased in plasma of HIV-1-infected subjects compared to uninfected subjects (median 4.69 pg/ml for HIV-1-infected subjects, median 4.69 pg/ml for uninfected subjects, p = 0.837) (B). Plasma IFNα levels were strongly associated with plasma IFN-I bioactivity in HIV-1-infected subjects (r = 0.711, p<0.001) (C). Plasma IP-10 was increased in plasma of HIV-1-infected subjects in comparison to uninfected subjects (median 538.2 pg/ml for HIV-1-infected subjects, median 132.6 pg/ml for uninfected subjects, p = 0.002) (D). Slight variations in sample sizes for different assays occur as results for some subjects were not available.