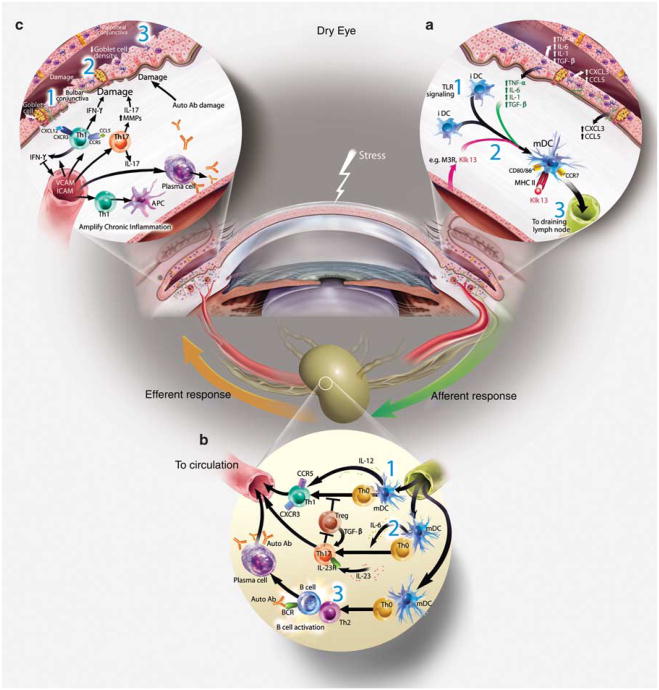
Figure 2.
The immunopathogenesis of Dry Eye disease. (a) Afferent autoimmune response: stress to the ocular surface triggers the initial events suggested to initiate autoimmunity. (1) Induction of proinflammatory factors (cytokines, chemokines, and matrix metalloproteinases (MMPs)) is a hallmark of ocular surface autoimmunity and may be initiated by stress signal transduction pathways or possibly by Toll-like receptor (TLR) signaling (after microbial infection or aberrant endogenous activation). Acute response cytokines, such as interleukin (IL)-1 α, IL-1 β, tumor necrosis factor (TNF)-α, and IL-6 are elevated in patients with Dry Eye. IL-1 and TNF-α amplify inflammation on the ocular surface through processes, such as activation of APCs. (2) Evidence suggests that APCs internalize autoantigen, for example, type III muscarinic receptor (M3R), Kallikrein (Klk)13, process and present immunogenic epitopes on MHCII, upregulate expression of costimulatory molecules, for example, CD80, and CD86 and (3) the chemokine receptor, CCR7, which directs the activated APCs to the draining cervical lymph node. (b) Efferent activation of autoreactive lymphocytes: the local cytokine milieu influences T-cell differentiation. (1) IL-12 present within the ocular surface tissues and produced by mature APCs, combined with interferon (IFN)-γ likely influences the activation and differentiation of autoreactive Th1 cells. (2) By contrast, elevated IL-6 in the presence of transforming growth factor (TGF)-β and IL-23 may skew differentiation toward Th17 cells. (3) B cells are also predicted to have a role in Dry Eye as shown in Sjögren's syndrome. In this scenario, Th2 cells provide help to autoreactive B cells and promote clonal expansion, somatic hypermutation, isotype switching, affinity maturation and plasma cell differentiation into autoantibody-secreting cells. Alternatively, B cells may be activated independently of T cells, for example, TLR and BAFF signaling. Efferent trafficking of autoreactive T cells to the ocular surface tissues is widely thought to be directed by adhesion molecules (e.g., LFA-1, VLA-4) and chemokine receptors (e.g., CCR5 and CXCR3) that respond to cognate ligands expressed on the ocular surface (e.g., ICAM-1, CCL5, and CXCL10). (c) Efferent effector function of autoreactive lymphocytes: Autoreactive Th1 and Th17 cells present during the immunopathogenesis of Dry Eye potentiate the chronic autoimmune response and have pathological consequences. For example, Th1 cells stimulate APCs to secrete proinflammatory cytokines and are a prominent source of interferon (IFN)-γ, which induces expression of a multitude of proinflammatory factors, pro-apoptotic proteins and causes direct tissue destruction. IFN-γ alters mucins on corneal epithelial cells that have devastating effects on ocular surface integrity. IFN-γ is linked to (1) epithelial cell apoptosis, (2) reduced goblet cell density, and (3) squamous metaplasia. IL-17 produced by infiltrating Th17 cells increases MMP3/9 expression and induces corneal epithelial barrier dysfunction. Furthermore, autoantibodies may bind to target antigens causing direct tissue destruction.