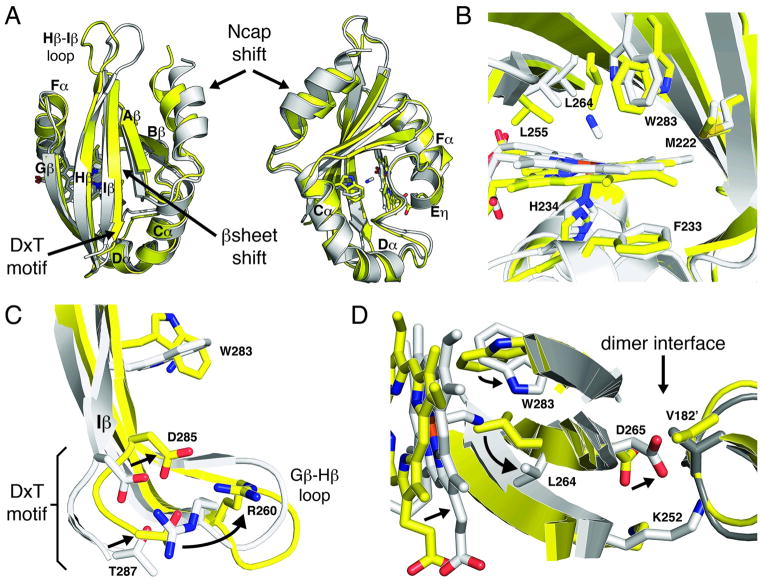
Figure 4. Conformational changes associated with ligand binding.
Structural alignment of ferric (yellow) and CN-bound (grey) Aer2 PAS domains. (A) The absence of ligand alters the PAS domain structure shifting the β sheet and Ncap helix. (B) The active site rearranges in the absence of ligand with the Trp283 side chain, which interacts with bound CN, rotating 90 degrees and the Leu264 side chain moving over the iron center. (C) Ligand-induced conformational changes propagate to the DxT motif, at the C-terminal end of the Iβ strand, and associated Gβ-Hβ loop. (D) A superposition of the CN-bound structure on the ferric dimer structure finds the CN-bound monomer is incompatible with the ferric dimer. Asp265, which is adjacent to Leu264 in Hβ, collides with Val182, located at the C-terminal end of the Ncap, across the dimer interface. Superimposed CN-bound PAS molecules are shown as light grey (left) and dark grey (right).