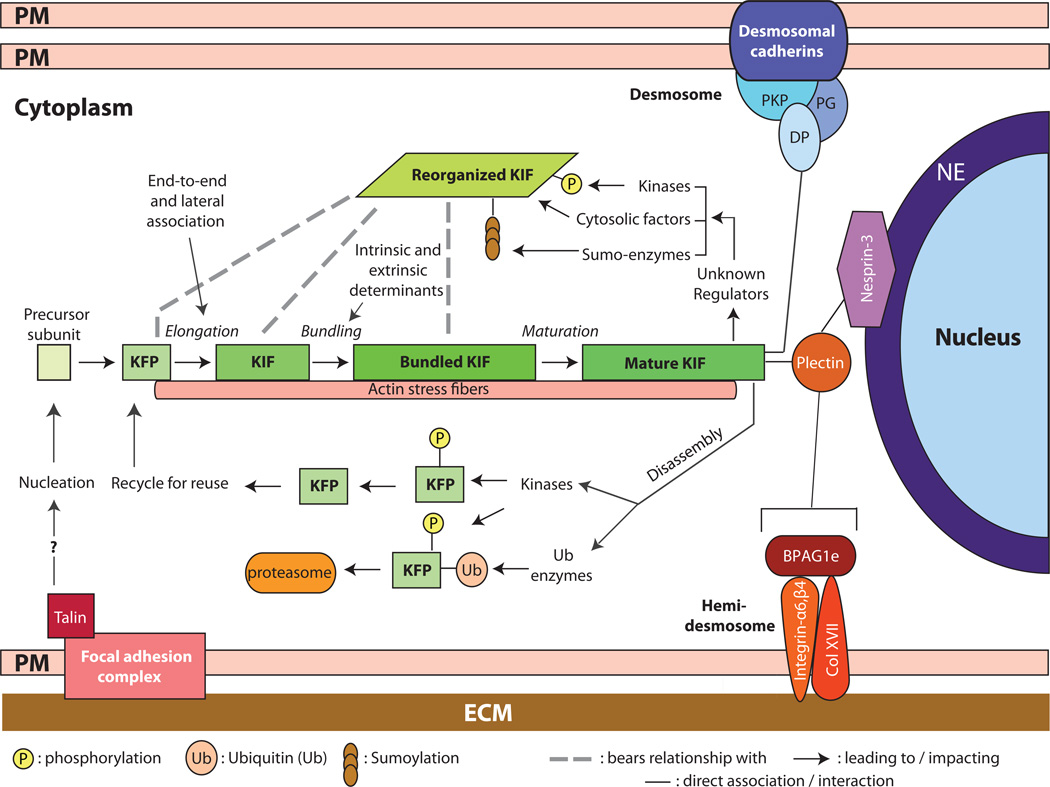
Figure 1.
Assembly, organization, and regulation of keratin intermediate filaments (KIFs). Live imaging studies in epithelial cells in culture show that keratin filament assembly is initiated at the periphery of the cell, near focal adhesions, and that newly formed filaments and their maturation into an organized network takes place in the context of a continuous centripetal flow with disassembly and turnover steps taking place near the nuclear envelope. The resulting “keratin cycle” is highly dependent on interactions with F-actin, with additional proteins, and on several types of post-translational modifications including phosphorylation, ubiquitination, sumoylation and (though not shown here) O-glycosylation. Most likely, the biological context dictates the rate of flow through this cycle. The figure also conveys that KIFs are attached at the surface of the nucleus (via a plectin/Nesprin-3 complex), at desmosome cell-cell adhesion sites, (via desmoplakin (DP), among other proteins), and at hemidemosome cell-matrix adhesions (via plectin and BPAG1e). Not shown here are the interactions with F-actin and microtubules. The structural support role of KIFs depends upon their organization as a crosslinked network that is fully integrated with other structural elements within and between epithelial cells. NE: nuclear envelope; PM: plasma membrane; ECM: extracellular matrix; DP: desmoplakin; PKP: plakophilin; KFP: keratin filament precursor; KIF: keratin intermediate filament.