Fig. 2. Association and glycosylation status of the purified A. gambiae AChE2 analyzed by SDS-PAGE and immunoblotting.
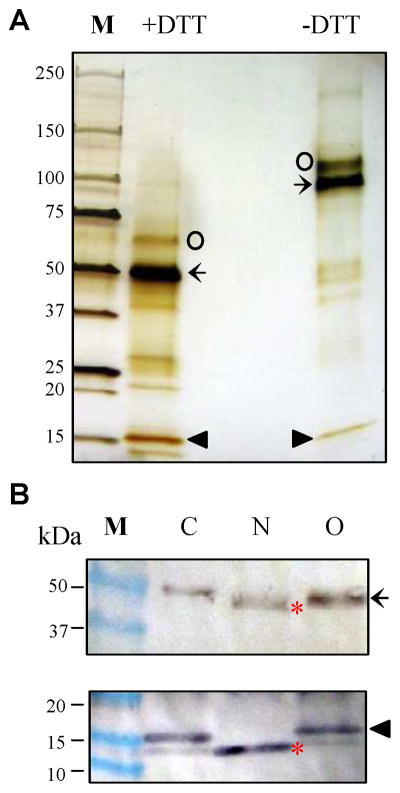
(A) The recombinant protein (435 ng) was treated with SDS sample buffer with or without DTT, separated on a precast 4–15% gradient gel, and visualized by silver staining. Intact AChE2 (67 kDa) and its cleavage products (51 and 16 kDa) are labeled by ○, ←, and ▲, respectively. (B) The purified enzyme (435 ng) was treated with buffer (lane C), PNGase F (lane N) or O-glycosidase (lane O), SDS sample buffer with DTT, separated on the gradient gel, and electrotransferred onto nitrocellulose membrane. Immunblot analysis was performed using diluted anti-(His)5 (upper panel) or anti-AChE2 (lower panel) serum the primary antibody. Sizes and positions of the Mr markers (lane M) are indicated on the left. After deglycosylation with PNGase F, the heavy and light chains (*) migrated faster than the untreated or O-glycosidase-treated ones.
