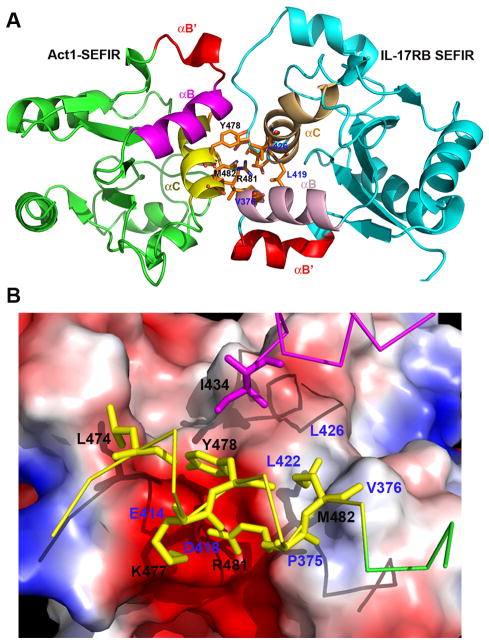
Fig. 4. A model of heterodimer of Act1-SEFIR and IL-17RB-SEFIR domains.
(A) Act1-SEFIR structure was modeled by SWISS-Model interface (http://swissmodel.expasy.org), using the crystal structure of IL-17RB-SEFIR as the template. Subsequent docking of Act1-SEFIR: IL-17RB-SEFIR was carried out with HADDOCK server (http://haddock.science.uu.nl), using identified key functional residues as constraints. Notice the helices αB′ (red) from both Act1-SEFIR and IL-17RB-SEFIR are exposed and not involved in the hetero-dimerization. (B) IL-17RB-SEFIR crystal structure is shown as electropotential surface, while residues on Act1-SEFIR are shown as sticks (yellow ones on αC and magenta ones onαB). The contact residues from Act1-SEFIR and IL-17RB-SEFIR are labeled in black and blue, respectively.