Abstract
Objective
To describe a mixed-methods approach to develop and test a basic behavioral science-informed intervention to motivate behavior change in three high-risk clinical populations. Our theoretically-derived intervention comprised a combination of positive affect and self-affirmation (PA/SA) which we applied to three clinical chronic disease populations.
Methods
We employed a sequential mixed methods model (EVOLVE) to design and test the PA/SA intervention in order to increase physical activity in people with coronary artery disease (post-percutaneous coronary intervention [PCI]) or asthma (ASM), and to improve medication adherence in African Americans with hypertension (HTN). In an initial qualitative phase, we explored participant values and beliefs. We next pilot tested and refined the intervention, and then conducted three randomized controlled trials (RCTs) with parallel study design. Participants were randomized to combined PA/SA vs. an informational control (IC) and followed bimonthly for 12 months, assessing for health behaviors and interval medical events.
Results
Over 4.5 years, we enrolled 1,056 participants. Changes were sequentially made to the intervention during the qualitative and pilot phases. The three RCTs enrolled 242 PCI, 258 ASM and 256 HTN participants (n=756). Overall, 45.1% of PA/SA participants versus 33.6% of IC participants achieved successful behavior change (p=0.001). In multivariate analysis PA/SA intervention remained a significant predictor of achieving behavior change (p<0.002, OR=1.66, 95% CI 1.22–2.27), controlling for baseline negative affect, comorbidity, gender, race/ethnicity, medical events, smoking and age.
Conclusions
The EVOLVE method is a means by which basic behavioral science research can be translated into efficacious interventions for chronic disease populations.
Keywords: Behavioral intervention, behavioral risk reduction, health behavior, mixed methods, translational research
In 2008, 63% of the 57 million people who died worldwide succumbed to non-communicable diseases and of these, an astounding 85% of people died from cardiovascular disease, diabetes, cancer or chronic respiratory diseases (World Health Organization, 2009). The “slow motion disaster” of chronic illness has become a public health crisis worldwide (Rosenbaum & Lamas, 2011). Behavior change holds the greatest untapped potential to reduce risk and extend life, but behavioral health promotion efforts remain greatly underleveraged. The lack of evidence-based approaches to effectively motivate long-term behavior change is a significant impediment in this effort (McGinnis, Williams-Russo, & Knickman, 2002).
Decades of research have been conducted to elucidate the fundamental bases of human behavior, yet few basic behavioral and social science findings have been translated into clinical interventions. Thus, there is enormous unmet potential to translate successes from basic research into applications that can improve outcomes in high-risk clinical populations; the use of a staged approach applying mixed methods may facilitate this process. To promote the translation of basic behavioral and social science into applications for clinical populations, a Broad Agency Announcement (BAA) was released in 2001 by the National Heart, Lung, and Blood Institute (NHLBI) of the National Institutes of Health (NIH). The announcement solicited applications to translate innovative, cutting-edge findings from basic behavioral and social science research to the prevention and management of heart, lung, blood and sleep diseases and disorders (National Institutes of Health, 2001). This paper describes the rationale, methods, and outcomes of one of the projects funded in response to this BAA, entitled the Cornell “Translational Behavioral Science Research Consortium” (TBSRC) conducted at Weill Cornell Medical College-New York Presbyterian Hospital in New York City.
TBSRC used a mixed-methods approach to develop and test a theoretically-derived intervention in three different chronic disease populations by translating positive affect and self-affirmation (PA/SA), constructs whose powerful effects on human behavior had been demonstrated in basic research studies in healthy people. We further describe a process model (EVOLVE) of sequential mixed methods that we used in designing the PA/SA intervention for use in people with chronic disease. The intervention was subsequently implemented and tested at an urban academic medical center in New York City in 756 participants in order to motivate increased physical activity in people with coronary artery disease who had undergone percutaneous coronary intervention (PCI) and people with asthma (ASM), and to increase medication adherence in African Americans (AA) with hypertension (HTN). It is our hope that this paper will provide researchers with one model for translating promising basic behavioral and social sciences research findings into behavioral interventions that can aid in managing high-risk clinical populations.
The Emerging Need for Mixed Methods
A myriad of public health issues—including ethnic, cultural and age disparities, seemingly intractable health behaviors, poor adherence to effective treatments and ongoing inequities in access to care—accompany the current chronic disease epidemic. Traditional quantitative methodology is limited in its ability to assess this complexity of public health problems. Mixed methods research comprises a participant-centered, culturally grounded set of techniques that employ, in tandem, methodologically rigorous quantitative and qualitative approaches in an integrated, theory-driven manner (Creswell, Klassen, Plano Clark, & Clegg Smith, 2011). By integrating qualitative and quantitative methods and findings into the study design, researchers can gain deeper insight into the participants’ point of view, explore complex social phenomena and effectively tailor intervention approaches.
Case Study: TBSRC
As an example of basic behavioral science translation, we identified two promising constructs for translational intervention development: positive affect and self-affirmation.
Positive affect
Positive affect (PA) is a mild feeling state induced by small events, such as receiving a small, unexpected gift, seeing a few minutes of comedy, or receiving a report of success on a small task (Ashby, Valentin, & Turken, 2002). PA has been shown to promote creativity and flexibility in problem-solving, help people to cope effectively and reduce defensiveness (Aspinwall, 1998; Estrada, Young, & Isen, 1994). Persons experiencing PA behave more cautiously and are less risk-prone than controls, even though they are more optimistic about being able to achieve a good outcome (Nygren, Isen, Taylor, & Dulin, 1996). Research that has specifically studied the impact of PA on health risks suggests that people can be encouraged to engage in healthier options or to take health precautions when they think that by so doing they can avoid dangerous risks to their health (Keller, 1999).
PA also increases specific aspects of motivation. Studies have shown that PA increases motivation and its cognitive components (Ilgen, Nebeker, & Pritchard, 1981; Kanfer, 1990). The cognitive components of motivation center on people’s valuation of the end result or reward, their expectation that achieving an intermediate goal (e.g., increasing physical activity) will help them achieve the ultimate goal (e.g., improved health), and their expectation that, if they work hard at something, that effort will help them to achieve the intermediate goal (e.g., physical activity maintenance will lead to improved fitness, which will make walking easier over time) (Erez & Isen, 2002). In addition, research has found that PA leads people to feel more intrinsically motivated and to find things more enjoyable (Isen & Reeve, 2002). Finally, a large body of literature indicates that people experiencing PA are more accepting of trying new things, and demonstrate greater interest in social and leisure activities (Lyubomirsky, King, & Diener, 2005).
Given this evidence, we believed that positive affect would help to motivate initiation and maintenance of healthful behavior changes in patients with chronic disease. First, we thought that induction of PA could help people view their own negative health behaviors as riskier than controls, and behave accordingly. Second, we believed that induction of PA could assist people with chronic illness to maintain a more optimistic view of behavior change, and be intrinsically motivated to make behavior changes. Finally, this body of work suggests that PA could lead people with chronic illness to be more open to new experiences, and thus more likely to initiate and maintain healthful behavior changes, such as physical activity, over time. For all of these reasons, we believed that positive affect would help people to overcome obstacles and be more successful than controls in initiating and maintaining behavior change over time.
Self-affirmation
Self-affirmation (SA) theory focuses on people’s sense of feeling morally and adaptively adequate, with meaning and value in life. In SA theory, the “self-system” is in a constant state of adaptation to maintain a moral sense and experience of the self, where the self is perceived as capable, good and able to make decisions with free choice (Steele, 1988). SA can be elicited when people recall accomplishments or important beliefs, making them feel proud. SA inductions have been shown to increase self-confidence, and enhance self-concept and resolve to overcome difficult situations (Steele, 1988).
People who are given personally-relevant health information may perceive it as threatening and process it in a biased way (Liberman & Chaiken, 1992). However, people who are self-affirmed respond more positively to information that challenges their beliefs (G. L. Cohen, Aronson, & Steele, 2000). In addition, women who received SA induction learned health information faster, were able link health behavior with adverse outcomes and felt more control over their behavior (Aspinwall, 1999). Similarly, it has also been shown that when people are presented with personally-threatening health information, SA improves their ability to process that information and increases behavioral intention (Sherman, Nelson, & Steele, 2000). For example, people who had SA induction rated themselves as being at higher risk for HIV and were more likely to purchase condoms and take educational materials (Sherman et al., 2000). Taken together, these studies indicate that SA induction could help people with chronic disease process new health information, link health behavior with an adverse outcome, enhance their sense of control and increase behavioral intention.
Given these findings, we believed that SA could be useful to people with chronic illness, particularly when receiving health information. Our trials employed SA procedures to help people with chronic disease overcome negative expectations they might have about their own ability to engage in a healthy behavior goal. Several reasons underlie our rationale for selecting SA. First, to the extent that people with chronic illness might feel threatened by receiving personally-relevant health information, we believed SA techniques could help them to overcome defensive processing of the information. Second, minority group members may view themselves as particularly vulnerable. SA techniques have been demonstrated to assist minority group members in overcoming stereotype threat (Steele & Aronson, 1995). Third, chronic illness may alter self-concept. SA techniques can enhance self-concept, and when performed prior to delivering threatening health messages, have been shown to assist people in processing self-relevant negative information (Aspinwall, 1999). Finally, people with chronic illness may have little or no experience with engaging in the health behaviors presented. Thus, they would have low self-efficacy for engaging in a behavior. We believed SA could improve confidence and success in making a behavior change.
The relationship between positive affect and self-affirmation
We hypothesized that PA/SA would have synergistic effects, because each has been found to increase peoples’ openness to information, including health information. The evidence was stronger for positive affect, which included enhanced motivation, flexibility of thinking and reduced risk taking. However, there was compelling evidence that SA could bolster health information processing, and importantly, enhance self-concept. Moreover, we believed that SA could augment our PA intervention and promote behavior change. SA addresses self-concept specifically and we believed it could also address the challenges that people with chronic disease might face in making health behavior changes. Finally, we believed that SA might also induce positive feelings and enhanced self-efficacy, thereby enabling and/or empowering behavior change.
Rationale for Selecting Cardiopulmonary Disease Participants
Behavioral change (e.g., smoking cessation, physical activity) has great potential to prevent chronic illness, reduce the burden of existing disease and decrease risk in patients with cardiopulmonary disease. We selected three high-risk patient groups with cardiopulmonary disease in order to test the combined PA/SA intervention.
Coronary artery disease and percutaneous coronary intervention (PCI)
The use of percutaneous coronary intervention for coronary artery disease, introduced in the early 1980s, has since increased over nine-fold and currently over 1.1 million Americans undergo the procedure annually (National Center for Health Statistics, 2010). After one year, 20–25% of PCI patients experience major morbidity or mortality (e.g., Javaid et al., 2007). Engaging in regular physical activity can significantly improve outcomes. For example, participation in ≥ one year of exercise-based cardiac rehabilitation reduces all-cause and cardiac mortality by 13% and 26%, respectively (Heran et al., 2011). Nonetheless, most PCI patients do not maintain changes in physical activity (Dolansky, Stepanczuk, Charvat, & Moore, 2010).
Asthma (ASM)
Asthma prevalence has more than doubled since 1980 and currently afflicts 8.2% of Americans (Akinbami, Moorman, & Liu, 2011). People with ASM are more likely to be overweight than the general population, although the mechanisms are not fully understood (Ford & Mannino, 2005). Many people with ASM are physically inactive because they are afraid of exacerbating their ASM symptoms (Mancuso et al., 2006). Chronic ASM can be precipitated or exacerbated by engaging in physical activity (Akinbami et al., 2011). Engaging in regular physical activity helps people with ASM maintain their respiratory functioning over time (Hallstrand, Bates, & Schoene, 2000). Conversely, physical inactivity leads to respiratory deconditioning and is associated with more exercise-induced asthma symptoms (Hallstrand et al., 2000).
Hypertension (HTN)
Hypertension prevalence has increased by 50% since 1980 (U.S. Census Bureau, 2001), and almost one-third of Americans suffer from the disease. HTN increases the risk of stroke, renal disease, myocardial infarction and death (Cushman, 2003). AAs have a higher prevalence compared with whites (Keenan & Rosendorf, 2011). Poor medication adherence is a major contributor to inadequate blood pressure control; half of people who take antihypertensive medications are non-adherent, and AAs are less likely to be adherent than whites (Monane et al., 1996). Blood pressure control significantly reduces cardiovascular morbidity and mortality (Officers, Coordinators for the, & Lipid-Lowering Treatment to Prevent Heart Attack, 2002), thus improving adherence among AAs with HTN holds great potential.
Specific Aims
Our primary goal was to motivate behavior change over one year in participants with high-risk cardiopulmonary disease. Specifically, we sought to: 1) increase physical activity in participants who had recently undergone PCI; 2) increase physical activity in participants with ASM; and 3) improve medication adherence in AA participants with HTN.
Methods
In order to translate combined PA and SA to behavior change, we employed a three-phase, mixed-methods approach whose acronym is EVOLVE—Explore Values, Operationalize and Learn, and eValuate Efficacy. EVOLVE includes a qualitative phase, a pilot phase and a randomized controlled trial (RCT) phase (see Figure 1). The studies were conducted with parallel design at New York Presbyterian Hospital-Weill Cornell Medical Center from 2003 to 2007. The Institutional Review Board approved the qualitative, pilot and RCT study phases for each of the three clinical populations. Participants in all study phases provided informed, written consent prior to enrollment.
Figure 1.
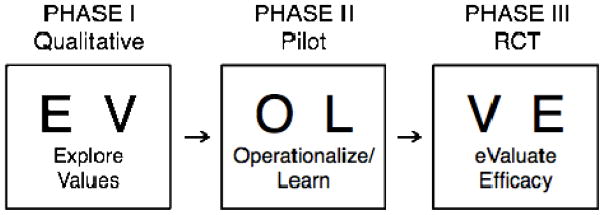
EVOLVE Method for Behavioral Intervention Development.
Participants
Eligible participants were ≥ 18 years old and English speaking. For the PCI study, eligible participants had undergone a recent PCI and received medical clearance from their physician to increase physical activity. We excluded people enrolled in other risk-reduction trials. For the ASM study, eligible participants had mild to moderate persistent ASM. In both the PCI and ASM studies, we excluded people with comorbidity that limited their ability to engage in or increase physical activity (e.g., debilitating medical conditions or musculoskeletal deficits). For the HTN study, eligible participants self-identified themselves as AA or black, had a diagnosis of HTN and were prescribed at least one antihypertensive medication.
Procedures
Phase I: Qualitative study to Explore Values
The specific aim of Phase I was to elucidate barriers to and facilitators of behavior change and identify important values, attitudes and beliefs about living with the chronic illness. The qualitative questions included items such as: How does [disease] affect your life?; What type of things do you do now that you may not have done before your [disease]?; and Do you have any advice for people with [disease]? (Charlson et al., 2007). Responses were used in the subsequent phases to help tailor the study methods and the intervention. In order to attain a maximally varied sample, participants were stratified according to racial/ethnic group for the PCI and ASM projects, with a goal of enrolling 1/3 AA, 1/3 Hispanic and 1/3 Caucasian participants. This approach enabled the identification of any specific variations in beliefs or perceived barriers that might be unique to one racial/ethnic group or another, while allowing the identification of patterns that could be common to all groups. Details of the qualitative methods are described elsewhere (Boutin-Foster, Ogedegbe, Ravenell, Robbins, & Charlson, 2007; Mancuso et al., 2006; Peterson et al., 2010).
Phase II: Pilot study to Operationalize and Learn
The aim of Phase II was to pilot test specific components of the modified intervention and study plan based on the qualitative results and to test the intervention delivery approach. A significant portion of Phase II was dedicated to iteratively refining the intervention and its delivery. PA/SA had never been “induced” in a clinical population, nor used in combination; therefore, we initially tested PA and SA individually. Once we had successfully determined the optimal approach to deliver each intervention (PA alone and SA alone), we then combined them. We also tested a control condition, and employed a parallel study design across the three clinical groups. Each study enrolled 40 patients into the following four arms: PA only (n=10), SA only (n=10), PA/SA combined (n=10), and control (n=10). Therefore, the sampling frame was identical, as was the intervention that was delivered.
We enrolled participants, administered baseline questionnaires, delivered the intervention, and then conducted follow-up a week later. During both interviews, we collected quantitative and qualitative data. We held weekly team meetings during the course of the 4-month pilot phase and reviewed participant and interviewer feedback. During this phase, we also developed detailed study protocols, which included staff training and fidelity monitoring, and procedures for screening and recruiting eligible participants.
Phase III: Randomized controlled trial to eValuate Efficacy
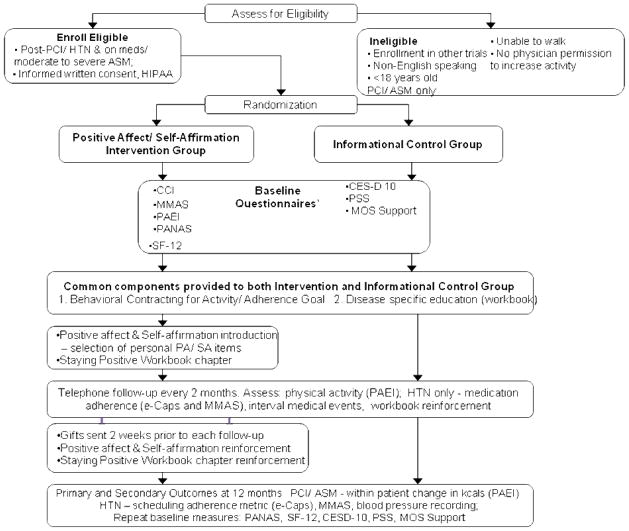
We conducted three RCTs with parallel study design between 2004 and 2007 in the three clinical chronic disease populations. A detailed description of the flow of participants from screening to completion of 12-month follow-up is depicted in the Study Flow Chart (Figure 2). Detailed methods are available (Charlson et al., 2007; Mancuso et al., 2012; Ogedegbe et al., 2012; Peterson et al., 2012).
Figure 2.
RCT Flow Chart
Participants
We approached participants and asked them to provide written, informed consent at primary care clinics within the New York Presbyterian-Weill Cornell Medical Center ambulatory network in the ASM and HTN trials. In the PCI trial we enrolled patients during index hospitalization at New York Presbyterian-Weill Cornell Medical Center. Inclusion/exclusion criteria were as described above.
Procedure
We randomized participants to the Informational Control (IC) or PA/SA Intervention with a randomization scheme devised by and known only to the study biostatistician. We blinded the treating physicians, the principal investigator, research coordinator and clinical outcome assessors.
IC group
Participants in the IC group received our informational workbook (developed in the qualitative phase) and behavioral contracting (Lorig & Fries, 1990) for a self-selected physical activity goal for physical activity (PCI and ASM) or medication adherence (HTN). Participants in the PCI and ASM groups also received a Yamax Digiwalker pedometer and logbook.
PA/SA intervention
Participants in the PA/SA group received the IC components (above) and the bimonthly PA/SA intervention by telephone and mail. To induce positive affect, we asked participants to “think about small things that make [them] feel good” and to take a moment to enjoy them each day. We also mailed small gifts (e.g., duffel bag, fleece blanket, umbrella) to induce PA two weeks prior to each follow-up. PA was also reinforced with an additional workbook chapter, entitled “Staying Positive.” To induce SA, we asked participants to recall “proud moments” when they found it difficult to adhere to their behavior goal. Details of the PA/SA intervention (Charlson et al., 2007) are contained in Table 1.
Table 1.
Intervention Component Details for RCT
| Theory | Time-point | Details |
|---|---|---|
| PA | Baseline |
|
|
|
||
| Bi-monthly |
|
|
|
|
||
| SA | Baseline |
|
|
|
||
| Bi-monthly |
|
Adapted from (Charlson et al., 2007)
Measures
We collected baseline demographics, including age, education and employment. We documented medical history, including the duration of index medical condition, the Charlson Comorbidity Index (Charlson, Pompei, Ales, & MacKenzie, 1987) and disease-specific measures (e.g., Seattle Angina Questionnaire (Spertus et al., 1995), ASM-related quality of life (Juniper et al., 1992)). All participants completed the following measures at baseline and 12 months: the Positive and Negative Affect Schedule (PANAS) (Watson, Clark, & Tellegen, 1988), Medical Outcomes SF-12 (Ware, Kosinski, & Keller, 1996), Center for Epidemiologic Studies Depression Scale (CES-D) (Andresen, Malmgren, Carter, & Patrick, 1994), Perceived Stress Scale (PSS) (S. Cohen, Kamarck, & Mermelstein, 1983), and the MOS Social Support (Sherbourne & Stewart, 1991).
Surveillance
Participants in both randomization groups received bimonthly follow-up calls from a trained research assistant. Follow-ups reinforced workbook content and assessed interval medical events and the health behavior. Interviewers employed a standardized script and we monitored treatment fidelity.
Outcomes
PCI and ASM
We measured physical activity in kcal/week using the Paffenbarger Physical Activity and Exercise Index (e.g., Paffenbarger, Wing, & Hyde, 1978), which has demonstrated validity and reliability (e.g., Jacobs, Ainsworth, Hartman, & Leon, 1993). The primary physical activity outcome was an increase in within-patient expenditure of ≥336 kcal/week from baseline to 12-months.
HTN
Participants in the HTN study received an electronic pill monitor (Medic-eCap®), the accepted standard for adherence assessment (Cramer, 1995). We asked participants to self-monitor one prescribed antihypertensive medication. Adherence rate was calculated as the number of times the pill bottle was opened divided by the scheduled number of doses per month. In the original trial, the primary outcome was a continuous variable, mean adherence at 12 months(Ogedegbe et al., 2012). For the analyses in this paper, we defined a dichotomous outcome variable in order to compare results across the three clinical populations. We considered mean adherence of ≥ 80% over 12 months to be “adherent.” This definition is commonly used in the HTN literature (e.g., Lee et al., 1996).
Sample size
Sample size calculations have been reported (Mancuso et al., 2012; Ogedegbe et al., 2012; Peterson et al., 2012). We required 242 participants for the PCI trial, 258 for the ASM trial and 256 for the HTN trial in order to have 90% power.
Statistical Analyses
We analyzed data using SAS version 9.3 and Stata 10, both for Windows. For continuous variables, we calculated means and standard deviations, and for categorical variables, we calculated counts and percentages. We used an intention-to-treat analysis, in which all participants with valid data were included. For those participants who were missing 12-month outcomes, we used the final time point prior to close-out, dropout, or death. We used logistic regression to assess the intervention effect, and calculated confidence intervals using the mid-p exact value (Wells, 2010). We also used univariate logistic regression to analyze the baseline and interval medical events as predictors of the main study outcomes (≥336 Kcal/week or ≥80% adherence at 12-months). We performed a series of logistic regression models on the combined trial data using the above dependent variables for physical activity and adherence. We adjusted all analyses for the following clinical and psychosocial variables: randomization group, age, baseline comorbidity, chronic disease group and baseline negative affect. No data were imputed.
Results
Phase I: Qualitative Study to Explore Values
Participant characteristics
Overall, 180 participants were enrolled in the qualitative phase (60 PCI, 60 ASM and 60 HTN). In the PCI group, the mean age was 64 ± 9 years, 48% were women, 33% were AA and 33% were Hispanic. 72% completed high school (Peterson et al., 2010). In the ASM group, the mean age was 45 ± 13 years, 88% were women, 47% were AA and 20% were Hispanic. Forty percent completed college and 47% were employed (Mancuso et al., 2006). All participants in the HTN study were AA, 92% were women and the mean age was 61 years (range 29–84). 88% completed high school (Boutin-Foster et al., 2007).
Applying qualitative findings
We made changes in three main areas: information and knowledge, intervention refinement, and recruitment/retention methodology (Table 2).
Table 2.
Qualitative Findings Applied to the Pilot Phase
| Methodology revisions | Cultural tailoring | |
|---|---|---|
| Information/knowledge | Develop informational workbooks:
|
|
| Intervention | Provide workbooks to control and intervention group | Identify population-specific core values for SA invention. |
| Recruitment/retention | Adjust recruitment strategy (e.g., enrolled immediately post-PCI) | Research staff of the same race/ethnicity as subjects |
Information and knowledge
We used our qualitative results to create culturally-relevant, disease-specific informational workbooks (Allegrante et al., 2004; Boutin-Foster et al., 2004; Mancuso, Sayles, Robbins, & Allegrante, 2010). The workbooks addressed the themes that arose for each of the specific disease groups in the qualitative phase. For example, many participants were not able to provide accurate information about their disease, its etiology, treatment and the role of behavior changes in risk reduction. Knowledge deficit about the importance of healthy behaviors in reducing risk and improving health could have been an important moderator of our subsequent pilot study and efficacy trials, confounding our results.
We were careful to ensure that we addressed disease- and population-specific issues that arose in the qualitative interviews in each of the workbooks, and used participant quotes to illustrate examples. For example, in the HTN group, a participant mentioned that AAs with HTN are particularly sensitive to salt. This participant’s quote was used, along with his suggestions for avoiding excess salt intake, including avoiding fast food at lunch and cooking with herbs and spices instead of salt. In the PCI study, we learned from participants that undergoing PCI was a “turning point, and a time when they were likely to reprioritize their lives” (Peterson et al., 2010), which helped them make successful behavior changes. This was incorporated into the PCI workbook. In the ASM group, participants noted that medical conditions, and asthma in particular, were a barrier to physical activity. Participants gave suggestions for physical activity, which we incorporated into the workbook, including knowing one’s limitations, slowing down or taking a break when symptoms arise and actively using medications (Mancuso et al., 2010).
Intervention refinement
First, to equalize knowledge and attention, we decided to provide the informational workbooks to both the intervention and control groups. Second, we identified core values from the qualitative interviews in order to induce SA that we tested in the subsequent pilot phase.
Recruitment and retention
Information obtained from the qualitative interviews allowed us to refine our recruitment and retention methods in the subsequent pilot phase. As noted above, participants thought that PCI was a turning point, and thus, the immediate post-recovery period was a “teachable moment” in this population. We decided to focus our recruitment efforts on enrolling participants early in their recovery period. We also learned that, when researchers recruited and interviewed participants of the same race or ethnic group, participants were more likely to consent and to report feeling more comfortable during interviews. In response, we hired diverse researchers for the subsequent pilot and RCT phases.
Phase II: Pilot Study to Operationalize and Learn
Participant Characteristics
A group of 131 participants consented and participated in the pilot phase (44 PCI, 40 ASM, 47 HTN). In the PCI group, 18% of participants were AA, 9% were Hispanic, 18% were multi-racial and 27% were female. In the ASM group, 40% of participants were AA, 35% were Hispanic and 85% were female. In the HTN group, all participants were AA and 57% were female.
Applying pilot findings
We made changes in two main areas as a result of the pilot study findings: intervention refinement and recruitment/retention methodology (Table 3).
Table 3.
Pilot Findings Applied to the RCT Phase
| Methodology revisions | Cultural tailoring | |
|---|---|---|
| Intervention |
|
|
| Recruitment/retention |
|
|
Intervention refinement
The investigative team met weekly throughout the course of the pilot phase. We tested and refined strategies for participant self-induction of PA and SA individually and then carefully considered the sequence of delivery of the combined intervention. We iteratively refined the wording of the intervention script during the 4-month pilot. We also developed intervention checks to assess the participants’ reactions to the intervention and its impact on the dependent variables. We reviewed participant responses with experts in PA (Isen) and SA (Steele and Aronson) theory in order to obtain their feedback in optimizing the delivery of the intervention.
Based on participant responses, the team explored whether the procedure for behavioral goal setting was specific enough; for example, early in the Pilot, we asked participants in the PCI and ASM pilots, “Do you think you can walk more each week? How many blocks more?” Late in the Pilot, the team decided to add behavioral contracting in order to delineate: 1) in what behavior a participant would engage; 2) how much they would perform; 3) when they would do it; and 4) how often (Lorig & Fries, 1990). The team decided to provide behavioral contracting to both randomization groups. We also tested the use of a pedometer and a recording logbook in the PCI and ASM participants in the pilot phase. Based on the pilot results, we added these components to the RCT, in order to provide participants with objective feedback and reinforcement. The team decided that both the intervention and control groups would receive pedometers. Finally, the team decided to add a chapter to the informational workbook for the intervention group only, emphasizing disease-specific and culturally relevant PA and SA.
Recruitment and retention
We worked with administrative staff at the enrollment sites to develop strategies to identify eligible participants using existing computer systems. During the pilot phase, we also developed qualitative questions and reviewed participant responses regarding length of follow-up, acceptability of the intervention and experience with the questionnaire battery. Further, we found that it was difficult to contact some of the participants during weekday/daytime hours and adjusted staff schedules accordingly.
Phase III: Randomized Controlled Trial to eValuate Efficacy
Participant characteristics
A total of 7,062 participants were screened, 2,391 were found to be eligible and 756 participants were enrolled in the RCT phase; 379 were randomized to the PA/SA group and 377 were randomized to the IC group. Overall, 94% (n=356) of PA/SA group and 93% (n=350) of IC group completed 12 months. Detailed consort diagrams for the 3 RCTs have been published (Mancuso et al., 2012; Ogedegbe et al., 2012; Peterson et al., 2012). For baseline demographics by disease group see Table 4.
Table 4.
RCT Demographic and Clinical Characteristics
| PCI | ASM | HTN | ||||
|---|---|---|---|---|---|---|
| IC (n=118) | PA/SA (n=124) | IC (n=128) | PA/SA (n=130) | IC (n=131) | PA/SA (n=125) | |
| Demographic characteristics | ||||||
| Age, mean (SD), yrs | 64 (11) | 62 (11) | 42 (12) | 42 (12) | 59 (12) | 57 (12) |
| Women | 30% | 31% | 73% | 76% | 77% | 82% |
| White race | 86% | 14% | 52% | 56% | - | - |
| AA race | 8% | 14% | 24% | 19% | 100% | 100% |
| Other race | 24% | 25% | ||||
| Hispanic ethnicity | 15% | 11% | 30% | 32% | 0 | 0 |
| Completed college | 53% | 57% | 60% | 62% | 56% | 60% |
|
| ||||||
| Clinical characteristics | ||||||
| Charlson Index | ||||||
| 0 | 27% | 24% | 0 | 0 | 16% | 30% |
| 1–3 | 50% | 53% | 98% | 99% | 61% | 46% |
| ≥ 4 | 23% | 23% | 2% | 1% | 23% | 24% |
| BMI | ||||||
| <25 | 28% | 23% | 33% | 30% | 36% | 37% |
| ≥ 25 to <30 | 36% | 42% | 25% | 32% | 22% | 13% |
| ≥ 30 | 36% | 35% | 42% | 38% | 42% | 50% |
|
| ||||||
| Psychosocial characteristics, mean (SD), score | ||||||
| Positive affect | 32 (9) | 33 (7) | 36 (9) | 35 (8) | 35 (8) | 35 (8) |
| Negative affect | 23 (9) | 23 (8) | 19 (7) | 20 (8) | 19 (17) | 19 (17) |
Note: For full demographic and clinical information for each of the trials, see (Mancuso et al., 2012; Ogedegbe et al., 2012; Peterson et al., 2012)
In the PCI study, the mean age was 63±11 years, 30% were women, 11% were AA and 13% were Hispanic. Sixty-nine percent were married and 55% completed college. Twenty-three percent had a Charlson Index ≥4 (indicating high burden of chronic disease), 75% were overweight or obese. In the ASM study, the mean age was 42±12 years, 75% were women, 22% were AA and 31% were Hispanic. Thirty-two percent were married and 61% completed college. One percent had a Charlson Index ≥4 and 67 % were overweight or obese. In the HTN study, the mean age was 58±12 years, 80% were women, 100% were AA and 3% were Hispanic. Twenty-five percent were married and 26% completed college. Twenty-three percent had a Charlson Index ≥4 and 64% were overweight or obese.
Primary outcome
Using logistic regression, we evaluated the effect of demographic and clinical variables and interval medical events on achieving the primary outcome in each of the three clinical populations (Table 5). When the trials were analyzed individually, participants in both the PCI and HTN studies who received the PA/SA intervention were significantly more likely to achieve successful behavior change at 12 months. PCI participants in the PA/SA group were twice as likely to increase their physical activity expenditure by ≥336 kcal/week (p=0.004, OR=2.01, 95% CI 1.19–3.39). Fifty-five percent of PA/SA participants met this outcome at 12 months, compared with 37% of controls (Peterson et al., 2012). Similarly, HTN participants in the PA/SA group were almost 1.9 times more likely to have an antihypertensive medication adherence rate of ≥ 80% as compared with controls (p=0.02, OR=1.87, 95% CI 1.03 – 3.40). ASM participants randomized to the PA/SA group were not more likely to increase their physical activity expenditure by ≥ 336 kcal/week compared with the IC control Group (p=0.23, OR=1.21, 95% CI 0.75–1.99). (Mancuso et al., 2012).
Table 5.
Univariate Predictors of Primary Study Outcome in the 3 Groups, OR (95% CI), N=756
| Independent variables | PCI (n=237) | ASM (n=258) | HTN (n=244) |
|---|---|---|---|
| Age | 0.99 (0.97, 1.01) | 1.03 (1.00, 1.05) | 1.00 (0.97, 1.02) |
| Male | 1.06 (0.61, 1.84) | 1.87 (1.06, 3.30) | 1.81 (0.92, 3.56) |
| < High school | 0.93 (0.52, 1.70) | 1.28 (0.50, 3.27) | 0.83 (0.46, 1.50) |
| Caucasian | 1.45 (0.75, 2.79) | 1.40 (0.86, 2.29) | -- |
| Black | 0.48 (0.20, 1.14) | 0.98 (0.54, 1.77) | -- |
| Latino | 1.75 (0.80, 3.85) | 1.24 (0.73, 2.10) | -- |
| High comorbidity (Charlson ≥4) | 0.75 (0.41, 1.39) | 2.29 (0.21, 25.52) | 0.98 (0.88, 1.09) |
| BMI ≥ 30 | 1.04 (0.61, 1.77) | 0.86 (0.52, 1.42) | 1.07 (0.59, 1.91) |
| CES-D ≥ 10 | 0.77 (0.46, 1.31) | 1.45 (0.88, 2.39) | 0.47 (0.24, 0.92) |
| Positive affect | 1.00 (0.97, 1.03) | 0.97 (0.95, 1.00) | 1.04 (1.00, 1.08) |
| Negative affect | 1.01 (0.98, 1.04) | 1.01 (0.98, 1.05) | 0.97 (0.93, 1.01) |
| Had any interval medical events | 0.27 (0.13, 0.59) | 0.89 (0.50, 1.56) | 0.28 (0.06, 1.25) |
| (Random)*(had medical events) | 0.33 (0.12, 0.93) | 0.87 (0.50, 1.54) | 0.67 (0.14, 3.19) |
| Self-efficacy for behavior goal (1–10) | 1.16 (0.97, 1.39) | 1.33 (1.05, 1.69) | 2.13 (1.20, 3.78) |
Cross trial analysis
Achieving the main trial outcome
We next evaluated the effect of the PA/SA intervention across the three study populations. Overall, 45.1% of PA/SA participants versus 33.6% of IC participants achieved successful behavior change (p=0.001, OR=1.63, 95% CI 1.21–2.19). In multivariate analysis, the PA/SA intervention remained a significant predictor of achieving successful behavior change (p<0.002, OR=1.66, 95% CI 1.22–2.27), controlling for negative affect, comorbidity, gender, race/ethnicity, interval medical events, smoking, age and chronic disease group (Table 6). In this analysis, people who were 75 years or older were significantly less likely to reach the main trial outcome (p=0.03, OR=0.48, 95% CI 0.24–0.95) as were people who experienced an interval medical event (p=0.003, OR=0.52, 95% CI 0.34–0.80).
Table 6.
Multivariate Predictors of Primary Study Outcome in the Combined Data, OR (95% CI), n=733
| OR (95% CI) | p-value | |
|---|---|---|
| PA/SA Intervention | 1.66 (1.22–2.27) | <0.002 |
| Age ≥ 75 years | 0.48 (0.24–0.95) | 0.03 |
| Male gender | 1.39 (0.97–1.99) | 0.07 |
| Comorbidity | 0.98 (0.91–1.05) | 0.53 |
| Interval medical event | 0.52 (0.34–0.80) | 0.003 |
| Smoking history | 0.21 (0.07–0.64) | 0.006 |
Controlling for randomization group, age, baseline comorbidity, chronic disease group and baseline negative affect
Achieving behavior maintenance
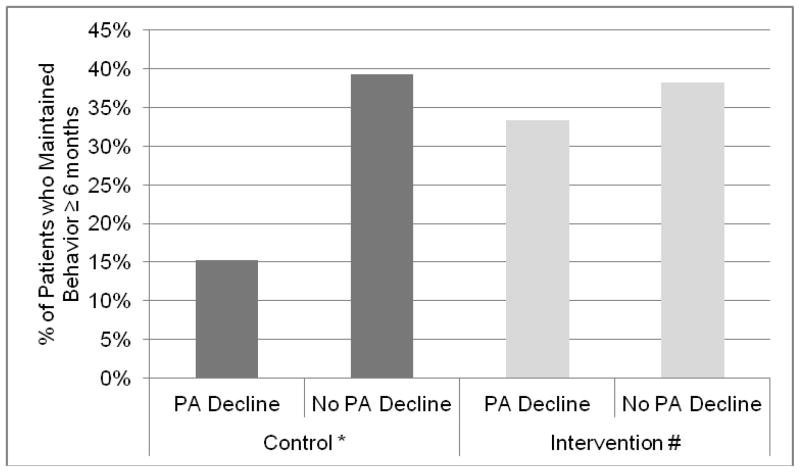
We next examined predictors of achieving behavior maintenance, a critical aspect of behavior change. For these analyses, we defined maintenance as maintaining the behavior for ≥ 6 months. Taking the baseline standard deviation of the population’s PANAS positive score of 8 points as a clinically important decline in positive affect from baseline to 12 months, we found that patients with this decline were significantly less likely to maintain behavior change (p<0.02, OR=0.49, 95% CI 0.27–0.90) in univariate analysis. In multivariate analysis (controlling for randomization group, age, baseline comorbidity, chronic disease group and baseline negative affect), those with a decline in positive affect were significantly less likely to achieve behavior maintenance (p=0.01, OR=0.29, 95% CI 0.11–0.78). In addition, we found that people who experienced an interval medical event were significantly less likely to achieve behavior maintenance (p=0.02, OR=0.59, 95% CI 0.37–0.93). In this model, the interaction between positive affect decline and randomization to the PA/SA group was associated with achieving maintenance of behavior change (p=0.09, OR=2.96, 95% CI 0.83–10.54). In other words, PA/SA intervention appeared to protect patients with declines in positive affect, enabling them to better maintain behavior change. We found that among patients with a decline in positive affect, 15% of those in the IC control group achieved maintenance compared to 33% in the PA/SA intervention group (p=0.046) (Figure 3). As shown in Figure 4, among patients with declines in positive affect, those in the PA/SA group faired significantly better with respect to physical activity outcomes compared with the IC control group (data displayed for the PCI and ASM trials). When we examined within-patient change in weekly kcal expenditure from baseline to 12 months among people with an eight-point decline in PANAS positive, those in the PA/SA intervention group had significantly higher expenditure compared to IC controls (742 ± 1361 vs. −842 ± 2452, p<0.02).
Figure 3.
Maintenance of behavior ≥ 6 months according to decline in PANAS positive score at 12 months by randomization group. *p=0.007 #p=0.59
Figure 4.
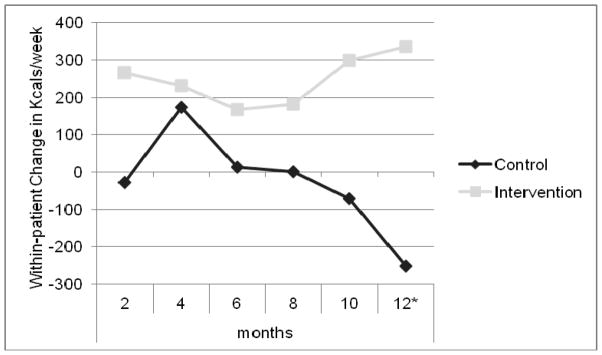
Median within-patient change in kcals/week for those with a decline in PANAS positive by randomization group (ASM and PCI). *p<0.02
Discussion
In this paper we present a systematic study design and procedures that have been tested in three different clinical populations and can be replicated in other efforts to translate promising basic behavioral science to clinical populations with chronic disease. We have described how the EVOLVE method provided a framework for translating the constructs of PA and SA from basic psychological science into an intervention approach that motivated behavior change in patients with chronic disease.
The EVOLVE method enabled us to design: 1) a core behavioral intervention, and 2) supportive elements that augmented the intervention and its implementation. Several points about the intervention are worth noting. First, the intervention itself was identical across the three disease groups and across cultural groups. We found that a range of important variables, including baseline demographic and clinical characteristics, including race/ethnicity, older age and comorbidity, did not independently predict the outcome in the individual RCTs (Table 5). Second, consistency in the delivery of the intervention is critical. For that reason, we developed a standardized script to deliver the core intervention. Third, during the pilot phase we reviewed participant responses to the intervention with experts in PA and SA theory; this was instrumental in modifying the intervention in a manner that enabled patients to link both PA and SA to their health behaviors goals.
The supportive elements that were added to the core intervention varied across the three trials. These included design elements such as disease-specific workbooks with participant quotes and vignettes drawn from the qualitative phase and research staff of the same race/ethnicity as participants. Intervention customization approaches such as these can enhance the reach and relevance of interventions in culturally diverse groups (Kreuter, Lukwago, Bucholtz, Clark, & Sanders-Thompson, 2003).
Our study had several notable strengths, including a large sample size (n=1,056), the use of sequential mixed methods and parallel study design in three separate chronic disease groups. There were also several limitations. First, we followed participants for 12 months. Although behavior change at one year represents an important step, longer-term studies are needed. Second, we tested the intervention in participants with cardiopulmonary disease, thus, generalizability to other disease groups is not yet established. However, we are currently testing the intervention with obese AAs and Hispanics, with promising preliminary results for physical activity, weight loss and health screening outcomes.
The cross-study analysis demonstrated that the PA/SA intervention is a significant predictor of behavior change in people with cardiopulmonary disease, controlling for negative affect, comorbidity, gender, interval medical events, smoking and age. Our analyses also revealed that people with cardiopulmonary disease who were ≥ 75 years or who had sustained an interval medical event were significantly less likely to achieve successful behavior change or maintain behavior change for ≥ 6 months. Nonetheless, in multivariate analysis PA/SA intervention remained the strongest predictor of achieving successful behavior change (OR=1.66) (Table 6). Our findings also demonstrate that the PA/SA intervention enabled people with declines in positive affect to better maintain behavior change (Figures 3 and 4). This is consistent with a recent cross sectional study that reported that higher positive affect was associated with greater levels of physical activity in young healthy women (Pasco et al., 2011). The implications are significant, given the many epidemiologic studies report that people reporting positive affect have significantly lower risk of mortality (Kubzansky & Thurston, 2007; Levy, Slade, Kunkel, & Kasl, 2002; Ostir, Markides, Black, & Goodwin, 2000). We hypothesize that physical activity mediates the relationship between positive affect and survival.
Although declines in positive affect may seem counterintuitive among people receiving a positive affect intervention, positive affect can be measured in different ways. In our RCTs, we used the 20-item PANAS measure, which is among the most widely used measures of positive and negative affect. The PANAS assesses “activated” positive affect. Indeed, most work in positive affect has focused on evaluating activated positive affect, that is, emotions such as feeling interested, proud, enthusiastic or determined. Conversely, the PANAS does not measure non-activated positive affect, such as feeling calm, at ease, content or satisfied. These emotions are distinctly different from negative affect (e.g., irritable, hostile, nervous or jittery). We hypothesize that the PANAS did not completely measure the impact of our positive affect intervention, because it does not assess non-activated positive affect, which may have been an important component of our intervention. Moreover, due to the nature of self-affirmation, after reflecting on proud moments, a logical result would be contentment and satisfaction with oneself. These emotions are consistent with non-activated positive affect and are not measured by the PANAS. Nonetheless, although activated positive affect decreased among some participants in the PA/SA intervention group, health behavior change was maintained (Figures 3 and 4).
There are several reasons why the EVOLVE method offers advantages in developing behavioral interventions for use in chronic disease. When an efficacious behavioral intervention in a controlled setting is implemented in real-world settings, effectiveness is usually less than original efficacy. This is because an intervention tested under controlled conditions often lacks relevance to the needs, requirements and inclinations of subcultural groups (Castro, Barrera, & Holleran Steiker, 2010). Cultural adaptations thus serve to increase the reach of an intervention; however, there are no fidelity standards for post-hoc intervention modification, and cultural adaptations of evidence-based interventions have produced mixed results (Griner & Smith, 2006). The EVOLVE method, which employs sequential mixed methods as an integral part of intervention development, is one strategy that can be used to create more broadly applicable evidence-based interventions, thus limiting the need for later adaptations.
We believe that the implications of the EVOLVE method and the findings we achieved in the linked trials are significant to behavior change efforts for two reasons. First, despite the promise of previous achievements in basic behavioral and social science research, there has been little systematic effort to translate these successes to clinical applications. The trials presented here demonstrate how the translation of two social psychological constructs, PA and SA, were utilized to foster clinically meaningful behavioral changes in three chronic disease populations. Second, the EVOLVE method can be applied to translate other basic behavioral and social science constructs from the laboratory to high-risk clinical populations.
Chronic disease prevention and management depend upon the adoption and maintenance of health behaviors, an area where basic behavioral and social research has much to offer. For example, cognitive psychologists have investigated “cognitive biases,” or patterns in the way people typically view the world based on individuals’ needs to organize and make sense of the environment. Studies show that people tend to categorize objects as starkly black and white, as dangerous or healthy, with inadequate consideration of exposure or dose (Redelmeier, Rozin, & Kahneman, 1993). Research on presentation of information has shown that when information on operative procedures is framed in terms of mortality rather than survival, patients are more anxious and less likely to elect surgery, even if other available choices result in less favorable outcomes (McNeil, Pauker, Sox, & Tversky, 1982). In addition, the relative effectiveness of gain-framed or loss-framed appeals depends, in part, on whether a behavior serves an illness-detecting or a health-affirming function (Rothman & Salovey, 1997). These findings may explain why patients sometimes appear to act against their own self-interests, such as exhibiting poor adherence to medical and lifestyle recommendations. They may also suggest why health care providers often fail to implement evidence-based practice guidelines, because cognitive and affective processes could also be expected to influence providers’ perceptions of a patient’s degree of risk and decisions regarding the appropriate course of treatment. These are just a few examples of promising basic science findings and how they might be applied in clinical populations.
The NIH Office of Behavioral and Social Sciences Research has advocated for formative and mixed methods research prior to beginning efficacy trials (Creswell et al., 2011). The success of this project, combined with NIH’s commitment to developing more powerful health-related behavioral interventions, has inspired a broad effort to promote the translation of basic behavioral and social sciences research into more effective behavioral interventions. For example, as part of the Obesity-Related Behavioral Intervention Trials (ORBIT) program, NIH scientists and researchers at seven sites are developing novel behavioral interventions to improve obesity-related behaviors (National Institutes of Health - Obesity Related Behavioral Intervention Trials (ORBIT), 2010). In addition, the NIH has recently released two funding announcements (PA 11-063 & PAR 11-346) to test novel and innovative interventions designed to promote adoption or maintenance of health behaviors; both include the option of using qualitative studies and mixed methods as part of the intervention development process (National Institutes of Health, 2010, 2011). All of these efforts, which build on the success of the Cornell TBSRC and its mixed-methods approach, are important parts of an emerging area of behavioral science: the translation of basic research findings into promising interventions that are useful in the treatment of clinical populations.
Acknowledgments
This research was supported by a grant from the National Institutes of Health, National Heart, Lung, and Blood Institute 1N01-HC-25096 Translational Behavioral Science Research Consortium.
Contributor Information
Janey C. Peterson, Division of Clinical Epidemiology and Evaluative Sciences Research, Department of Medicine, and Center for Integrative Medicine, Weill Cornell Medical College
Susan Czajkowski, Behavioral Medicine Scientific Research Group, Division of Prevention and Population Sciences, National Heart, Lung, and Blood Institute, National Institutes of Health.
Mary E. Charlson, Division of Clinical Epidemiology and Evaluative Sciences Research, Department of Medicine, and Center for Integrative Medicine, Weill Cornell Medical College
Alissa R. Link, Division of Clinical Epidemiology and Evaluative Sciences Research, Department of Medicine, and Center for Integrative Medicine, Weill Cornell Medical College
Martin T. Wells, Department of Biological Statistics and Computational Biology and Department of Statistical Sciences, Cornell University
Alice M. Isen, Division of Marketing, Johnson Graduate School of Management, Cornell University;
Carol A. Mancuso, Department of Medicine, Hospital for Special Surgery
John P. Allegrante, Department of Health and Behavior Studies, Teachers College, and Department of Sociomedical Sciences, Mailman School of Public Health, Columbia University
Carla Boutin-Foster, Division of Clinical Epidemiology and Evaluative Sciences Research, Department of Medicine, and Center for Integrative Medicine, Weill Cornell Medical College.
Gbenga Ogedegbe, Department of Medicine and Center for Healthful Behavior Change, New York University School of Medicine.
Jared B. Jobe, Behavioral Medicine Scientific Research Group, Division of Prevention and Population Sciences, National Heart, Lung, and Blood Institute, National Institutes of Health
References
- Akinbami LJ, Moorman JE, & Liu X. Asthma Prevalence, Health Care Use, and Mortality: United States, 2005–2009. Centers for Disease Control and Prevention; 2011. [PubMed] [Google Scholar]
- Allegrante JP, Charlson ME, Isen AM, Peterson JC, Ravenell KL, Robbins L. Living with heart disease: Taking control after angioplasty. New York: Joan and Sanford I. Weill Medical College of Cornell University; 2004. [Google Scholar]
- Andresen EM, Malmgren JA, Carter WB, Patrick DL. Screening for depression in well older adults: evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale) Am J Prev Med. 1994;10(2):77–84. [PubMed] [Google Scholar]
- Ashby F, Valentin V, Turken U. The effects of positive affect and arousal on working memory and executive attention: neurobiology and computational models. In: Moore S, Oaksford M, editors. Emotional Cognition: From Brain to Behaviour. Amsterdam: John Benjamins; 2002. pp. 245–287. [Google Scholar]
- Aspinwall LG. Rethinking the role of positive affect and self-regulation. Motivation and Emotion. 1998;23(1):1–32. [Google Scholar]
- Aspinwall LG. Introduction of Section: Persuasion for the Purpose of Cancer Risk Reduction: Understanding Responses to Risk Communications. JNCI Monographs. 1999;1999(25):88–93. doi: 10.1093/oxfordjournals.jncimonographs.a024216. [DOI] [PubMed] [Google Scholar]
- Boutin-Foster C, Ogedegbe G, Ravenell JE, Robbins L, Charlson ME. Ascribing meaning to hypertension: a qualitative study among African Americans with uncontrolled hypertension. Ethnicity & Disease. 2007;17(1):29–34. [PubMed] [Google Scholar]
- Boutin-Foster C, Ogedegbe GO, Ravenell JE, Allegrante JP, Charlson ME, Isen AM, Robbins L. Living with hypertension: Taking control. New York: Joan and Sanford I. Weill Medical College of Cornell University; 2004. [Google Scholar]
- Castro FG, Barrera M, Jr, Holleran Steiker LK. Issues and challenges in the design of culturally adapted evidence-based interventions. Annu Rev Clin Psychol. 2010;6:213–239. doi: 10.1146/annurev-clinpsy-033109-132032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlson ME, Boutin-Foster C, Mancuso CA, Peterson JC, Ogedegbe G, Briggs WM, Allegrante JP. Randomized controlled trials of positive affect and self-affirmation to facilitate healthy behaviors in patients with cardiopulmonary diseases: rationale, trial design, and methods. Contemporary Clinical Trials. 2007;28(6):748–762. doi: 10.1016/j.cct.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- Cohen GL, Aronson J, Steele CM. When beliefs yield to evidence: Reducing biased evaluation by affirming the self. Personality and Social Psychology Bulletin. 2000;26(9):1151–1164. [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–396. [PubMed] [Google Scholar]
- Cramer JA. Microelectronic systems for monitoring and enhancing patient compliance with medication regimens. Drugs. 1995;49(3):321–327. doi: 10.2165/00003495-199549030-00001. [DOI] [PubMed] [Google Scholar]
- Creswell J, Klassen A, Plano Clark V, Clegg Smith K. Best practices for mixed methods research in the health sciences. National Institutes of Health, Office of Behavioral and Social Sciences Research; 2011. Retrieved from http://obssr.od.nih.gov/scientific_areas/methodology/mixed_methods_research/index.aspx. [Google Scholar]
- Cushman WC. The burden of uncontrolled hypertension: morbidity and mortality associated with disease progression. [Review] Journal of clinical hypertension. 2003;5(3 Suppl 2):14–22. doi: 10.1111/j.1524-6175.2003.02464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolansky MA, Stepanczuk B, Charvat JM, Moore SM. Women’s and men’s exercise adherence after a cardiac event. Research in gerontological nursing. 2010;3(1):30–38. doi: 10.3928/19404921-20090706-03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erez A, Isen AM. The influence of positive affect on the components of expectancy motivation. J Appl Psychol. 2002;87(6):1055–1067. doi: 10.1037/0021-9010.87.6.1055. [DOI] [PubMed] [Google Scholar]
- Estrada CA, Young MJ, Isen AM. Positive affect influences creative problem solving and reported source of practice satisfaction in physicians. Motivation and Emotion. 1994;18:285–299. [Google Scholar]
- Ford ES, Mannino DM. Time trends in obesity among adults with asthma in the United States: findings from three national surveys. J Asthma. 2005;42(2):91–95. [PubMed] [Google Scholar]
- Griner D, Smith TB. Culturally adapted mental health intervention: A meta-analytic review. Psychotherapy. 2006;43(4):531–548. doi: 10.1037/0033-3204.43.4.531. [DOI] [PubMed] [Google Scholar]
- Hallstrand TS, Bates PW, Schoene RB. Aerobic conditioning in mild asthma decreases the hyperpnea of exercise and improves exercise and ventilatory capacity. Chest. 2000;118(5):1460–1469. doi: 10.1378/chest.118.5.1460. [DOI] [PubMed] [Google Scholar]
- Heran BS, Chen JM, Ebrahim S, Moxham T, Oldridge N, Rees K, Taylor RS. Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane database of systematic reviews. 2011;(7):CD001800. doi: 10.1002/14651858.CD001800.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilgen DR, Nebeker DM, Pritchard RD. Expectancy theory measures: An empirical comparison in an experimental situation. Organizational Behavior and Human Performance. 1981;28:189–223. [Google Scholar]
- Isen AM, Reeve JM. Positive affect promotes intrinsic motivation. Ithaca: 2002. [Google Scholar]
- Jacobs DR, Jr, Ainsworth BE, Hartman TJ, Leon AS. A simultaneous evaluation of 10 commonly used physical activity questionnaires. Medicine and science in sports and exercise. 1993;25(1):81–91. doi: 10.1249/00005768-199301000-00012. [DOI] [PubMed] [Google Scholar]
- Javaid A, Steinberg DH, Buch AN, Corso PJ, Boyce SW, Pinto Slottow TL, Waksman R. Outcomes of coronary artery bypass grafting versus percutaneous coronary intervention with drug-eluting stents for patients with multivessel coronary artery disease. Circulation. 2007;116(11 Suppl):I200–206. doi: 10.1161/CIRCULATIONAHA.106.681148. [DOI] [PubMed] [Google Scholar]
- Juniper EF, Guyatt GH, Epstein RS, Ferrie PJ, Jaeschke R, Hiller TK. Evaluation of impairment of health related quality of life in asthma: development of a questionnaire for use in clinical trials. Thorax. 1992;47(2):76–83. doi: 10.1136/thx.47.2.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanfer R. Motivation theory and industrial and organizational psychology. In: Dunnette MD, Hough LM, editors. Handbook of Industrial and Organizational Psychology. Vol. 1. Palo Alto: Consulting Psychologists Press; 1990. pp. 75–170. [Google Scholar]
- Keller PA. The effect of affect-based dissonance versus cognition-based dissonance on motivated reasoning and health-related persuasion. J Exper Psychol. 1999;5:302–313. [Google Scholar]
- Kreuter MW, Lukwago SN, Bucholtz RD, Clark EM, Sanders-Thompson V. Achieving cultural appropriateness in health promotion programs: targeted and tailored approaches. Health Education & Behavior. 2003;30(2):133–146. doi: 10.1177/1090198102251021. [DOI] [PubMed] [Google Scholar]
- Kubzansky LD, Thurston RC. Emotional Vitality and Incident Coronary Heart Disease: Benefits of Healthy Psychological Functioning. Arch Gen Psychiatry. 2007;64(12):1393–1401. doi: 10.1001/archpsyc.64.12.1393. [DOI] [PubMed] [Google Scholar]
- Lee JY, Kusek JW, Greene PG, Bernhard S, Norris K, Smith D, Wright JT., Jr Assessing medication adherence by pill count and electronic monitoring in the African American Study of Kidney Disease and Hypertension (AASK) Pilot Study. American journal of hypertension. 1996;9(8):719–725. doi: 10.1016/0895-7061(96)00056-8. [DOI] [PubMed] [Google Scholar]
- Levy BR, Slade MD, Kunkel SR, Kasl SV. Longevity increased by positive self-perceptions of aging. J Pers Soc Psychol. 2002;83(2):261–270. doi: 10.1037//0022-3514.83.2.261. [DOI] [PubMed] [Google Scholar]
- Liberman A, Chaiken S. Defensive Processing of Personally Relevant Health Messages. Personality and Social Psychology Bulletin. 1992;18(6):669–679. [Google Scholar]
- Lorig K, Fries JF. The arthritis help book: A tested self-management program for coping with your arthritis. Reading, MA: Addison-Wesley; 1990. [Google Scholar]
- Lyubomirsky S, King L, Diener E. The benefits of frequent positive affect: does happiness lead to success? Psychol Bull. 2005;131(6):803–855. doi: 10.1037/0033-2909.131.6.803. [DOI] [PubMed] [Google Scholar]
- Mancuso CA, Choi TN, Westermann H, Wenderoth S, Hollenberg JP, Wells MT, Charlson ME. A randomized trial to increase physical activity in asthma patients through positive affect and self-affirmation. Archives of Internal Medicine. 2012;172 doi: 10.1001/archinternmed.2011.1316. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancuso CA, Sayles W, Robbins L, Allegrante JP. Novel use of patient-derived vignettes to foster self-efficacy in an asthma self-management workbook. Health Promotion Practice. 2010;11(1):44–53. doi: 10.1177/1524839907309865. [DOI] [PubMed] [Google Scholar]
- Mancuso CA, Sayles W, Robbins L, Phillips EG, Ravenell K, Duffy C, Charlson ME. Barriers and facilitators to healthy physical activity in asthma patients. Journal of Asthma. 2006;43(2):137–143. doi: 10.1080/02770900500498584. [DOI] [PubMed] [Google Scholar]
- McGinnis JM, Williams-Russo P, Knickman JR. The case for more active policy attention to health promotion. [Review] Health Aff (Millwood) 2002;21(2):78–93. doi: 10.1377/hlthaff.21.2.78. [DOI] [PubMed] [Google Scholar]
- McNeil BJ, Pauker SG, Sox HC, Jr, Tversky A. On the elicitation of preferences for alternative therapies. New England Journal of Medicine. 1982;306(21):1259–1262. doi: 10.1056/NEJM198205273062103. [DOI] [PubMed] [Google Scholar]
- Monane M, Bohn RL, Gurwitz JH, Glynn RJ, Levin R, Avorn J. Compliance with antihypertensive therapy among elderly Medicaid enrollees: the roles of age, gender, and race. American journal of public health. 1996;86(12):1805–1808. doi: 10.2105/ajph.86.12.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Center for Health Statistics. Health, United States, 2009: With Special Feature on Medical Technology. Hyattsville, MD: 2010. [PubMed] [Google Scholar]
- National Institutes of Health - Obesity Related Behavioral Intervention Trials (ORBIT) Translating ideas into interventions: The process of developling behavioral interventions. San Diego, CA: 2010. [Google Scholar]
- National Institutes of Health. Translational behavioral science research consortia. Department of Health and Human Services; 2001. Retrieved from http://www.nhlbi.nih.gov/funding/inits/archive/baa02-07.htm. [Google Scholar]
- National Institutes of Health. Translating basic behavioral and social science discoveries into interventions to improve health-related behaviors (R01) Department of Health and Human Services; 2010. Retrieved from http://grants.nih.gov/grants/guide/pa-files/PA-11-063.html. [Google Scholar]
- National Institutes of Health. Interventions for health promotion and disease prevention in Native American populations (R01) Department of Health and Human Services; 2011. Retrieved from http://grants.nih.gov/grants/guide/pa-files/PAR-11-346.html. [Google Scholar]
- Nygren TE, Isen AM, Taylor PJ, Dulin J. The influence of postive affect on the decsion rule in risk situations: Focus on outcome (and especially avoidance of loss) rather than probability. Organizational Behavior and Human Decision Processes. 1996;66:59–72. [Google Scholar]
- Officers A Coordinators for the ACRGTA Lipid-Lowering Treatment to Prevent Heart Attack T. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) JAMA. 2002;288(23):2981–2997. doi: 10.1001/jama.288.23.2981. [DOI] [PubMed] [Google Scholar]
- Ogedegbe GO, Boutin-Foster C, Wells MT, Allegrante JP, Isen AM, Jobe JB, Charlson ME. A randomized controlled trial of positive affect intervention and medication adherence in hypertensive African Americans. Archives of Internal Medicine. 2012;172 doi: 10.1001/archinternmed.2011.1307. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostir GV, Markides KS, Black SA, Goodwin JS. Emotional well-being predicts subsequent functional independence and survival. J Am Geriatr Soc. 2000;48(5):473–478. doi: 10.1111/j.1532-5415.2000.tb04991.x. [DOI] [PubMed] [Google Scholar]
- Paffenbarger RS, Jr, Wing AL, Hyde RT. Physical activity as an index of heart attack risk in college alumni. Am J Epidemiol. 1978;108(3):161–175. doi: 10.1093/oxfordjournals.aje.a112608. [DOI] [PubMed] [Google Scholar]
- Pasco JA, Jacka FN, Williams LJ, Brennan SL, Leslie E, Berk M. Don’t Worry, be Active: Positive Affect and Habitual Physical Activity. Australian and New Zealand Journal of Psychiatry. 2011;45(12):1047–1052. doi: 10.3109/00048674.2011.621063. [DOI] [PubMed] [Google Scholar]
- Pérez-Perdomo R, Pérez-Cardona C, Disdier-Flores O, Cintrón Y. Prevalence and Correlates of Asthma in the Puerto Rican Population: Behavioral Risk Factor Surveillance System, 2000. Journal of Asthma. 2003;40(5):465–474. doi: 10.1081/jas-120018713. [DOI] [PubMed] [Google Scholar]
- Peterson JC, Allegrante JP, Pirraglia PA, Robbins L, Lane KP, Boschert KA, Charlson ME. Living with heart disease after angioplasty: A qualitative study of patients who have been successful or unsuccessful in multiple behavior change. Heart & Lung. 2010;39(2):105–115. doi: 10.1016/j.hrtlng.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson JC, Charlson ME, Hoffman Z, Wells MT, Wong SC, Hollenberg JP, Allegrante JP. Randomized controlled trial of positive affect induction to promote physical activity after percutaneous coronary intervention. Archives of Internal Medicine. 2012;172 doi: 10.1001/archinternmed.2011.1311. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redelmeier DA, Rozin P, Kahneman D. Understanding patients’ decisions. Cognitive and emotional perspectives. JAMA. 1993;270(1):72–76. [PubMed] [Google Scholar]
- Rosenbaum L, Lamas D. Facing a “slow-motion disaster”--the UN meeting on noncommunicable diseases. N Engl J Med. 2011;365(25):2345–2348. doi: 10.1056/NEJMp1112235. [DOI] [PubMed] [Google Scholar]
- Rothman AJ, Salovey P. Shaping perceptions to motivate healthy behavior: The role of message framing. Psychological Bulletin. 1997;121:3–19. doi: 10.1037/0033-2909.121.1.3. [DOI] [PubMed] [Google Scholar]
- Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med. 1991;32(6):705–714. doi: 10.1016/0277-9536(91)90150-b. [DOI] [PubMed] [Google Scholar]
- Sherman D, Nelson L, Steele C. Do messages about health risks threaten the self? Increasing the acceptance of threatening health messages via self-affirmation. Personality and Social Psychology Bulletin. 2000;26(9):1046–1058. [Google Scholar]
- Spertus JA, Winder JA, Dewhurst TA, Deyo RA, Prodzinski J, McDonell M, Fihn SD. Development and evaluation of the Seattle Angina Questionnaire: a new functional status measure for coronary artery disease. J Am Coll Cardiol. 1995;25(2):333–341. doi: 10.1016/0735-1097(94)00397-9. [DOI] [PubMed] [Google Scholar]
- Steele C. The psychology of self-affirmation: Sustaining the integrity of the self. In: Berkowitz L, editor. Advances in experimental social psychology. Vol. 21. New York: Academic press; 1988. pp. 261–302. [Google Scholar]
- Steele C, Aronson J. Stereotype threat and the intellectual test performance of African Americans. Journal of Personality and Social Psychology. 1995;69(5):797–811. doi: 10.1037//0022-3514.69.5.797. [DOI] [PubMed] [Google Scholar]
- U.S. Census Bureau. The Hispanic Population in the United States: Population Characteristics, March 2000. Washington, DC: 2001. Retrieved from http://www.census.gov/population/www/socdemo/hispanic/ho00.html. [Google Scholar]
- Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54(6):1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Wells M. Optimality Results for mid p-values. IMS Collections. Borrowing Strength: Theory Powering Applications - A Festschrift for Lawrence D Brown. 2010;6:184–198. [Google Scholar]
- World Health Organization. Global health risks: mortality and burden of disease attributable to selected major risks. Geneva: 2009. Retrieved from http://www.who.int/healthinfo/global_burden_disease/GlobalHealthRisks_report_full.pdf. [Google Scholar]