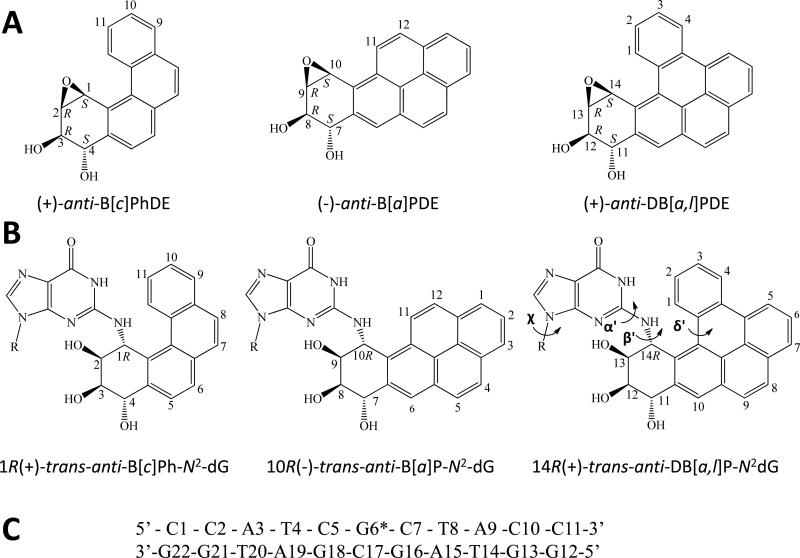
Figure 1.
(A) Structures of the S,R,R,S anti-PAH diol epoxides. These react with guanine in DNA by trans-addition to the 1 position of (+)-anti-B[c]PhDE, the 10 position of (–)-anti-B[a]PDE, and the 14 position of (+)-anti-DB[a,l]PDE to form the stereochemically defined adducts with the same absolute configurations defined in (B). The sequence of the modified 11-mer duplex is shown in (C), where G6* denotes the site of the modified guanine. The torsion angles α' (N1-C2-N2-C14), β' (C2-N2-C14-C13), and δ'(C15-C17-C20-C1) are indicated for the DB[a,l]P-dG adduct.