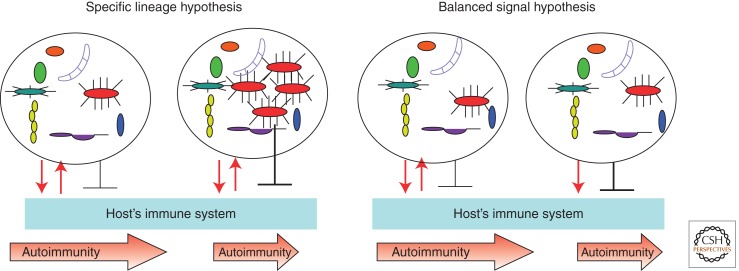
Figure 3.
The two hypotheses explain how microbes could provide protection against autoimmunity. The “specific lineage hypothesis” predicts that in genetically predisposed animals or humans, microbiota could provide signals that induce antimicrobial effectors that are neutralized by microbial inhibitory signals (reciprocating red arrows). As a result, the microbiota stays in a homeostatic relationship with the host (leftmost panel) and the disease progresses independently of microbiota. When a specific microbial lineage is expanded (next panel), it blocks the development of autoimmunity. It does so to improve its own odds of staying in this expanded state by suppressing the host’s inflammatory and adaptive responses. Autoimmunity is quenched as a side effect. The “balanced signal hypothesis” predicts that the host’s interactions with microbiota are independent of the precise microbiota composition and that the host’s genetics plays a critical role in the conversation with microbes. Whereas a balanced host response to commensals and the commensals’ effort to reduce this response do not affect disease development (middle right panel), the inability of the host to control the microbiota properly (such as in mice lacking PRR signaling because of a knockout of the adaptor molecule MyD88) (Wen et al. 2008) (rightmost panel) results in the dominance of negative signaling provided by the microbiota and reduction of autoimmunity. Both hypotheses predict that tolerizing effects would be lost in germ-free conditions.