Figure 6.
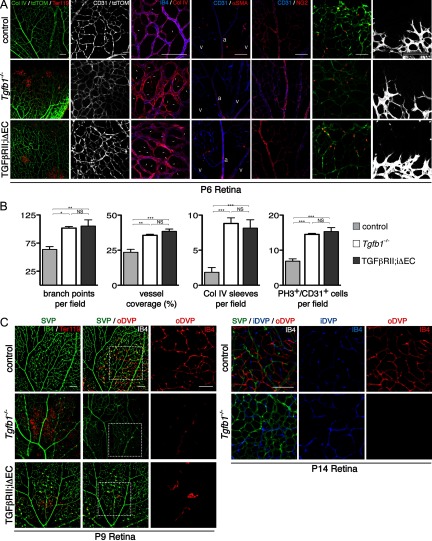
Absence of Tgfb1, and inhibition of TGFβ signaling in retinal endothelial cells recapitulate the vascular abnormalities observed in the Itgb8 mutants. A, Representative images of retina flat-mounts of control, Tgfb1−/− and endothelial-specific Tgfbr2 mutant (TGFβRII;iΔEC) P6 retinas. TGFβRII;iΔEC mutant retinas were generated using animals homozygous for the Tgfbr2-flox allele bearing also a Pdgfb promoter-regulated Cre-ER-T2 transgene. Cre recombination was induced by intragastric tamoxifen injections (50 μg) at P1, P2, and P3. Loss of TGFβ1 or TGFβRII results in hemorrhage at P6 as illustrated by extravascular red blood cells (Ter119) in the mutant retinas (left panels). Endothelial cells, visualized by endothelial cell-specific expression of tdTomato (Pdgfb-CreERTM2-mediated expression of recombination marker tdTomato from Rosa26RtdTomato;AI14 allele) and/or CD31 labeling illustrate enhanced vascular branching density in P6 Tgfb1−/− and TGFβRII;iΔEC mutant retinas. Localization of vascular endothelia (IB4) and collagen IV illustrate increased presence of collagen IV sleeve segments lacking endothelial cells (IB4-negative; arrows) in Tgfb1−/− and TGFβRII;iΔEC mutants. The relative distribution of α-smooth muscle actin (SMA) and NG2-expressing pericytes compared with CD31-expressing endothelial cells in control compared with mutants suggests that pericyte coverage is normal in the mutants. The same panels document reasonably normal arterial (a) and venous (v) differentiation in mutants. Colabeling with anti-CD31 and the tdTomato recombination reporter (to colabel endothelial cells) and phosphohistone3 (PH3) illustrate an increased density of proliferating endothelial cells in P6 Tgfb1−/− and TGFβRII;iΔEC mutant retinas. Images of the angiogenic front in control and mutant P6 retinas indicate that the morphologies and numbers of endothelial tip cells and their filopodia are not obviously perturbed in the Tgfb1−/− or endothelial-specific Tgfbr2 (TGFβRII;iΔEC) mutants. B, Panels present quantification of changes in retinal vascular development at P6 as a result of Tgfb1−/− deletion or endothelial cell-specific deletion of Tgfbr2. Results demonstrate that as a result of loss of TGFβ1 or impaired TGFβRII function, there are increases in vascular branch point density, the area of retina covered by vascular endothelial cells, the number of collagen IV sleeve segments lacking endothelial cell coverage, and the number of proliferating endothelial cells. ANOVA p-values: branch points per field, p < 0.0056; vessel coverage (%), Col IV sleeves per field, and PH3+/CD31+ cells per field, p < 0.0001. Tukey's subgroup analysis: *p < 0.05, **p < 0.005, ***p < 0.0005, NS = not significant; N = 9 (combined controls), N = 3 (Tgfb1−/−, TGFβRII;iΔEC). Error bars represent SEM. C, Representative images of flat-mounts of control, Tgfb1−/− and endothelial-specific Tgfbr2 (TGFβRII;iΔEC) mutant P9 and P14 retinas. Endothelial-specific Tgfbr2 mutant retinas were analyzed following tamoxifen administration (50 μg) on P5 and P6, retinal vascular phenotypes were analyzed on P9 by immunostaining vessels (IB4; green) and red blood cells (Ter119). Confocal optical slices were taken from the superficial vascular plexus (SVP, green), the inner deep vascular plexus (iDVP; blue), and the outer deep vascular plexus (oDVP, red) as illustrated in diagram in Figure 1. At P9, absence of TGFβ1 or acute loss of TGFβRII from endothelial cells leads to disruption of the SVP with corresponding leakage of red blood cells. At P9 formation of the outer DVP is dramatically inhibited in the absence of TGFβ1 or TGFβ receptor 2 in retinal endothelial cells. Right panels in P9 retina image sets are higher-magnification insets of outer DVP from boxed areas in middle panels. At P14 absence of TGFβ results in continued failure of formation of the outer deep vascular plexus. The inner deep vascular plexus does form in the mutant with abnormally thickened vessels, similar to the phenotypes in the Itgb8 mutant. At P14, TGFβRII;iΔEC mutants were sickly and obviously smaller than littermate controls, and so were not analyzed. Scale bars, 100 μm.