Abstract
Purpose
Patients with neuromuscular scoliosis are at increased risk of neurological deficit post-operatively, but are a difficult population on whom to perform neurophysiological monitoring. We look here at a 7-year sample of our practice in the monitoring of neuromuscular patients.
Methods
A retrospective chart review was performed for 109 patients who underwent correction of neuromuscular scoliosis within our institution between 2005 and 2011.
Results
Of 109 patients who were identified, intraoperative monitoring was attempted in 66 cases. In eight cases (13 %), no reliable monitoring could be achieved and was therefore abandoned. On nine occasions, there was a significant drop in at least one modality intraoperatively. None of these nine suffered any clinically observable neurological deficit post-operatively. Of the 109 patients, 2 had clinically detectable deficits post-operatively, both of whom had undergone normal intraoperative monitoring.
Conclusions
The two patients with observable deficit had their instrumentation left in situ after discussion with them and/or parents. Spinal cord monitoring in this population is possible but potentially unreliable. Surgeons will need to carefully consider the use of monitoring in their management of this challenging population.
Keywords: Neuromuscular, Scoliosis, Intraoperative, Neurophysiological, Monitoring, Spinal
Introduction
Neuromuscular scoliosis occurs in patients with one or more of a wide range of neurological or muscular diseases, including cerebral palsy, post-infective or post-traumatic encephalopathy, poliomyelitis, myelomeningocele, spinal muscle atrophy, muscular dystrophies and myopathies as well as those who have suffered a spinal cord injury. The precise incidence of scoliosis due to neuromuscular causes depends on the underlying condition and its severity, but ranges from 25 to 90 % [1].
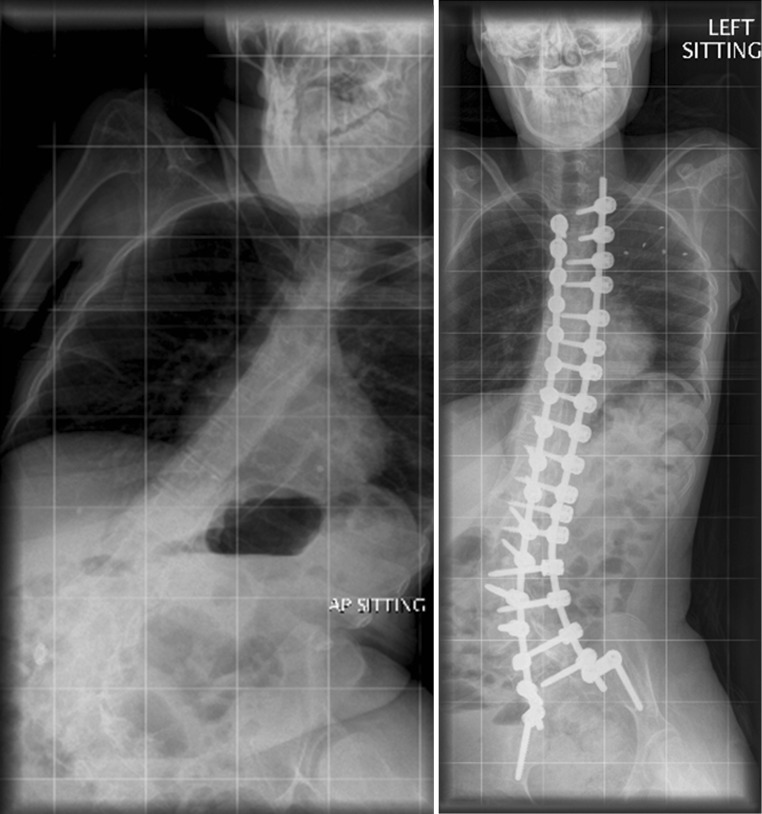
Beyond any cosmetic concerns, as scoliosis progresses there are considerable implications for the patient in terms of worsening sitting balance, respiratory function, costo-pelvic impingement (and resultant pain), and loss of upper limb function (if present) as they strive to remain upright. Corrective surgery generally results in good caregiver satisfaction [2], as well as greater perceived patient comfort and sitting ability [3]. Unfortunately, this patient population is particularly prone to a high rate of post-operative complications [3] as a direct result of their underlying health issues. One of the most feared complications of scoliosis correction surgery, regardless of aetiology, is that of paralysis; and, again, neuromuscular patients are at a greater risk of neurological deficit post-operatively as compared to those with idiopathic curves [4]. This is, to an extent, a result of the often severe and stiff curves that can occur with these conditions (Fig. 1).
Fig. 1.
A pre- and post-operative radiograph for a patient with scoliosis secondary to Rett syndrome
Intraoperative monitoring using Somatosensory Evoked Potentials (SSEPs) has long been accepted as a useful tool for the assessment of neurological status during spinal surgery, allowing for immediate remedial action to be taken in the theatre in reaction to any perceived change in cord status [5]. The use of transcranial electric motor evoked potentials (TceMEPs) has also been widely adopted, to allow for monitoring of the dorsal and ventral columns during procedures and the adoption of multiple monitoring modalities has been a natural evolution [6].
Sadly, even in modern practice, it is clear that monitoring does not detect all potential spinal cord damage, with the largest study, encompassing the whole gamut of spinal pathology (albeit with self-reported data) to date indicating that only 40 % of post-operative cord injuries were heralded by a change in neurophysiological monitoring [7]. Those with impaired neuromuscular function present considerable challenges in obtaining reliable baseline monitoring [8], further limiting the accuracy of these techniques in this patient group.
Methods
We reviewed the practice at a major spinal service with extensive experience in paediatric deformity correction. We performed a retrospective chart analysis on those patients undergoing corrective surgery for neuromuscular scoliosis between 2005 and 2011. Our practice during this time predominantly utilised SSEPs for the monitoring of patients, stimulating the posterior tibial nerve and using scalp electrodes to record the resultant impulses. Fears over the perceived potential to initiate epileptic seizures precluded the majority of these patients from undergoing TceMEPS.
Patients listed for the theatre for whom intraoperative monitoring was desired were sent for pre-operative SSEP assessment by the neurophysiologists, and if a reliable, reproducible recording could be obtained then the patient would go on to undergo intraoperative monitoring.
Where there was a significant change in monitoring, in this case defined in SSEPs as a drop relative to baseline amplitude below 50 % or the reduction of TceMEPs below 10 % of baseline, the neurophysiologist reassessed the correct positioning of the electrodes, the anaesthetic regime, blood pressure, and patient positioning. Finally, the surgeon was notified of the monitoring change, who would decide what, if any, further action would be taken.
Results
A total of 109 patients (56 males and 53 females) with neuromuscular diagnoses underwent operative intervention during the time period in question. Their ages at the time of surgery ranged from 5 to 26, mean age 14.3 (SD 4.9 years).
There were 61 patients who had cerebral palsy with varying degrees of severity [12 mild/moderate cases corresponding to Gross Motor Function Classification System (GMFCS) grades I–IV; 49 severe cases with whole body involvement (GMFCS grades V–VI)]; 12 had Duchenne muscular dystrophy, 9 had spinal muscular atrophy, 7 had Rett syndrome, and 23 had rarer diagnoses including limb girdle muscular dystrophy, Becker’s muscular dystrophy, hereditary motor and sensory neuropathy, Friedreich’s ataxia, myasthenia gravis and Ullrich congenital muscular dystrophy.
Spinal cord monitoring using at least one modality was attempted in 66 of these patients.
In the remaining 43 patients, 35 patients were initially listed for surgery without monitoring, and a further 8 underwent surgery without monitoring because the neurophysiology team was unable to achieve any reliable SSEPs at pre-operative assessment.
Of the 66 who proceeded to undergo intraoperative monitoring, 8 patients had to have their monitoring abandoned on the day of surgery when the neurophysiology team was unable to achieve reliable baseline SSEP readings in theatre.
In 43 patients, reliable SSEPs were achieved, and were the sole monitoring modality attempted, and in 15 patients reliable SSEPs and MEPs were achieved. MEPs were only attempted in a few patients who did not have a history of epilepsy due to concerns over initiating seizures. Although the technology and personnel were available during the period in question, the use of MEPs as routine was not established and so they were not attempted in all those patients who would currently be seen as eligible.
These 58 patients were able to be monitored throughout their intraoperative course. There were nine intraoperative monitoring (IOM) events in nine patients. The remainder of the patients had entirely stable monitoring throughout the duration of the operation.
Of the patients who had an IOM event, in five cases the monitoring recovered with standard non-surgical actions being taken as detailed previously. Of the four patients with a residual deficit in neuromonitoring, three lacked the ability to comply with a Stagnara wake-up test, and so the decision was taken to proceed with the surgery regardless of the monitoring results. The remaining patient complied with and passed a wake-up test. None of the patients who had an intraoperative monitoring event developed any post-operative deficit that was detectable on clinical examination, or reported by parents and/or carers following surgery.
There were, however, 2 patients of the 66 monitored who developed demonstrable post-operative neurological deficits. One patient with whole body involvement cerebral palsy stopped all spontaneous leg movement and developed a neuropathic bladder, and one patient with Duchenne muscular dystrophy developed L4 paresthesia and weakness bilaterally, immediately post-operatively. Both of these patients underwent monitoring, and both had undergone entirely normal SEPs throughout the procedure.
After discussions with the parents, in both cases the corrective instrumentation was left in situ.
See Tables 1 and 2 for tabular breakdown by diagnosis of successful monitoring.
Table 1.
Monitoring in cerebral palsy
| Degree of cerebral palsy | Mild/moderate (GMFCS I–IV) | Severe (GMFCS V–VI) |
|---|---|---|
| No monitoring planned | 3 | 16 |
| No monitoring achievable pre-operative | 0 | 6 |
| No monitoring achievable intraoperative | 1 | 2 |
| Monitoring achieved | 8 | 25 |
Table 2.
Monitoring in other significant neuromuscular diagnoses
| Diagnosis | Duchenne muscular dystrophy | Spinal muscular atrophy | Rett syndrome |
|---|---|---|---|
| No monitoring planned | 4 | 2 | 4 |
| No monitoring achievable pre-operative | 1 | 0 | 0 |
| No monitoring achievable intraoperative | 1 | 1 | 0 |
| Monitoring achieved | 6 | 6 | 3 |
Discussion
The topic of spinal cord monitoring in these patients has been a controversial one for decades. Several authors have demonstrated that monitoring these patients is possible [9, 10]. In our opinion, the most interesting finding is that in our retrospective analysis there is no apparent pattern to the utilisation of intraoperative neurophysiological monitoring. For example, those patients with severe, whole body involvement cerebral palsy are just as likely to undergo spinal cord monitoring as not. These patients had no useful voluntary movement or continence, and so the question must be asked precisely as to what neurological function we had aimed to preserve.
This underlines the lack of clarity of its precise role and the wide variation in practice between different surgeons. Our ability to obtain reproducible SSEPs in 75 % of patients with severe, whole body involvement cerebral palsy compares well with published literature [11], and lends credence to the belief that the majority of these patients can be monitored, if so desired. There remains the risk of false negatives in neuromuscular scoliosis [12] as well as in the adult population undergoing severe deformity correction [13].
It is our reaction to IOM events that raises the most significant questions about our approach to spinal cord monitoring. In none of the cases that we reviewed over the 7-year period did a change in monitoring make any difference to the surgical plan of action. Several patients underwent a change of anaesthetic management, or had their monitoring leads adjusted, but in no case was any instrumentation removed or corrective manoeuvre reversed as a result of alteration in neuromonitoring. In each case, the decision was made that the potential benefits of better sitting balance and a straighter spine outweighed any risk to continuing.
We must be clear in our practice as to the utility of monitoring, and as to what we aim to achieve by it, and we hope that this will remind all those who undertake scoliosis correction in this challenging patient population that the possibility of significant spinal cord injury as a direct results of surgical intervention must be considered and planned for. If intraoperative neuromonitoring is to continue to be a valid tool, then prior to using it one must carefully consider the actions to be taken in the event of an IOM event.
Conclusion
Monitoring is a challenging prospect in this patient population. The fact that it has proven feasible, though not necessarily reliable, in our review merely underlines this fact. This does not mean that we should abandon the use of intraoperative monitoring in any patient with a neuromuscular diagnosis. In those patients who have neurological function to be preserved in the form of movement, continence or protective sensation, we should absolutely continue to try and preserve this with all tools available to us. But in those without useful function, perhaps we should consider carefully as to why we are requested the use of monitoring and what we hope to achieve.
Conflict of interest
None.
References
- 1.Sarwark J, Sarwahi V. New strategies and decision making in the management of neuromuscular scoliosis. Orthop Clin N Am. 2007;38:485–496. doi: 10.1016/j.ocl.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 2.Jones KB, Sponseller PD, Shindle MK, et al. Longitudinal parental perceptions of spinal fusion for neuromuscular spine deformity in patients with totally involved cerebral palsy. J Pediatr Orthop. 2003;23:143–149. [PubMed] [Google Scholar]
- 3.Comstock CP, Leach J, Wenger DR. Scoliosis in total-body involvement cerebral palsy: analysis of surgical treatment and patient and caregiver satisfaction. Spine. 1998;23:1412–1425. doi: 10.1097/00007632-199806150-00022. [DOI] [PubMed] [Google Scholar]
- 4.Fehlings MG, Kelleher MO. Intraoperative monitoring during spinal surgery for neuromuscular scoliosis. Nat Clin Pract Neurol. 2007;3:318–319. doi: 10.1038/ncpneuro0502. [DOI] [PubMed] [Google Scholar]
- 5.Nuwer MR, Dawson EG, Carlson LG, et al. Somatosensory evoked potential spinal cord monitoring reduces neurologic deficits after scoliosis surgery: results of a large multicenter survey. Electroencephalogr Clin Neurophysiol. 1995;96:6–11. doi: 10.1016/0013-4694(94)00235-D. [DOI] [PubMed] [Google Scholar]
- 6.DiCindio S, Theroux M, Shah S, et al. Multimodality monitoring of transcranial electric motor and somatosensory-evoked potentials during surgical correction of spinal deformity in patients with cerebral palsy and other neuromuscular disorders. Spine. 2008;28(16):1851–1856. doi: 10.1097/01.BRS.0000083202.62956.A8. [DOI] [PubMed] [Google Scholar]
- 7.Eccher M. Intraoperative neurophysiologic monitoring: are we really that bad? J Clin Neurophysiol. 2012;29(2):157–159. doi: 10.1097/WNP.0b013e31824ff6d0. [DOI] [PubMed] [Google Scholar]
- 8.DiCindio S, Theroux M, Shah S, Miller F, Dabney K, Brislin RP, Schwartz D. Multimodality monitoring of transcranial electric motor and somatosensory-evoked potentials during surgical correction of spinal deformity in patients with cerebral palsy and other neuromuscular disorders. Spine (Phila Pa 1976) 2003;28(16):1851–1855. doi: 10.1097/01.BRS.0000083202.62956.A8. [DOI] [PubMed] [Google Scholar]
- 9.Ecker ML, Dormans JP, Schwartz DM, et al. Efficacy of spinal cord monitoring in scoliosis surgery in patients with cerebral palsy. J Spinal Disord. 1996;9:159–164. doi: 10.1097/00002517-199604000-00013. [DOI] [PubMed] [Google Scholar]
- 10.Padberg AM, Russo MH, Lenke LG, et al. Validity and reliability of spinal cord monitoring in neuromuscular spinal deformity surgery. J Spinal Disord. 1996;9:150–158. doi: 10.1097/00002517-199604000-00012. [DOI] [PubMed] [Google Scholar]
- 11.DiCindio S, Theroux M, Shah S, Miller F, Dabney K, Brislin RP, Schwartz D. Multimodality monitoring of transcranial electric motor and somatosensory-evoked potentials during surgical correction of spinal deformity in patients with cerebral palsy and other neuromuscular disorders. Spine. 2003;16:1851–1856. doi: 10.1097/01.BRS.0000083202.62956.A8. [DOI] [PubMed] [Google Scholar]
- 12.Ashkenaze D, Mudiyam R, Boachie-Adjei O, et al. Efficacy of spinal cord monitoring in neuromuscular scoliosis. Spine. 1993;18:1627–1633. doi: 10.1097/00007632-199309000-00010. [DOI] [PubMed] [Google Scholar]
- 13.Quraishi NA, Lewis SJ, Kelleher MO, et al. Intraoperative multimodality monitoring in adult spinal deformity: analysis of a prospective series of one hundred and two cases with independent evaluation. Spine. 2009;34(14):1504–1512. doi: 10.1097/BRS.0b013e3181a87b66. [DOI] [PubMed] [Google Scholar]