Abstract
Objective Describe the clinical course and outcomes of patients with primary acinic cell carcinoma (ACC) of the parotid gland with skull base invasion or metastasis.
Design Retrospective case series (1995–2011) at a single institution.
Results Ten patients met study criteria. Mean and median time from initial diagnosis of parotid ACC to development of skull base disease were 14.6 and 10.2 years, respectively. Two patients demonstrated skull base disease on initial presentation. Those who pursued further treatment after developing disease at the skull base underwent surgery (4/7), stereotactic radiosurgery (4/7), or external beam radiation (3/7). The 10-year Kaplan-Meier estimated overall survival after initial diagnosis of parotid ACC was 80%. Once skull base invasion occurred, 2-year estimated overall survival was 50%.
Conclusion Although primary ACC of the parotid generally caries an excellent prognosis, tumor control with cranial base disease is difficult and the majority of patients present with late aggressive recurrences. Our observations underscore the importance of long-term follow-up in this patient group.
Keywords: acinic cell carcinoma, salivary tumors, skull base, cranial base
Introduction
Acinic cell carcinoma (ACC) is a relatively rare salivary malignancy and represents ~3 to 11% of all adult parotid cancers.1,2 Originally classified as a benign neoplasm given its generally indolent course, it was first recognized as a malignant tumor by Buxton et al in 1953. Surgical excision with the goal of complete tumor removal—consisting of a superficial parotidectomy at minimum, avoiding simple tumor enucleation—is considered by many to be a standard initial treatment for resectable tumors. Overall, given the rarity of the disease and retrospective nature of available data, guidelines for total parotidectomy, elective neck dissection, facial nerve sacrifice, and postoperative adjuvant management of ACC have not been universally agreed upon.
Although disease characteristics such as locoregional spread and metastasis have been well described for more common salivary neoplasms,3 ACC has received less attention. Descriptions of primary parotid ACC with skull base invasion/metastasis have been limited to case reports.4,5,6,7,8 Treatment strategies for these patients, including the role of radiotherapy (fractionated or single fraction), chemotherapy, and aggressive surgical management, remain unclear.1,2,9,10,11,12 Herein, we describe the clinical presentation, treatment strategies, and characteristics of disease progression for a series of 10 consecutive patients evaluated for primary or recurrent ACC of parotid gland origin with skull base involvement.
Materials and Methods
After institutional review board approval (09-003999), a retrospective chart review at a tertiary academic referral center was conducted using the institutional electronic medical record. All patients who were diagnosed with parotid ACC and had skull base involvement were included. Data were collected with respect to presenting symptomatology, treatment strategies, and outcomes. Review of all pathology specimens was performed by pathologists at our institution at the time of surgery.
Results
Over the past 16 years, a total of 87 patients with a history of primary or recurrent ACC of the parotid gland were evaluated at our institution. Of these, 10 patients (mean age at initial ACC diagnosis 52.0 years, range 18 to 78 years, five women) developed involvement of the cranial base at some point in their clinical course (Table 1). Two patients had skull base disease on presentation: one with advanced unresectable ACC who was referred for palliative therapy and one who underwent initial treatment at an outside institution. Eight patients developed skull base disease after initial treatment of parotid ACC: one was initially treated at our institution, whereas the remaining patients were referred to us after developing recurrent disease. The mean and median follow-up periods from initial diagnosis were 16.5 and 13.7 years, respectively.
Table 1. Patients with Skull Base Involvement by Acinic Cell Carcinoma of Parotid Origin.
| Patient | Age at original diagnosis | Gender | Skull base disease at initial presentation? | Type of initial surgery | Grade | Extracapsular extension | Margins at initial excision | Initial adjuvant therapy | Time to first recurrence (years) | Time to development of skull base disease (years) | Location of skull base disease | Treatment of skull base disease | Cervical metastases? | Distant metastases? |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 18 | F | No | Total parotidectomy | 2 | No | Positive | None | 4 | 12 | Glenoid fossa, infratemporal fossa, Foramen ovale, Meckel's cave, cavernous sinus, sphenoid sinus, clivus, cerebellum | Extended parotidectomy, partial mandibulectomy, fibular free flap (FFF). External beam and stereotactic radiation. | − | + |
| 2 | 76 | M | No | Superficial parotidectomy | DNA | DNA | DNA | None | 64 | 10.1 | Masticator space, temporal fossa | External beam radiation | + | − |
| 3 | 23 | F | No | Superficial parotidectomy | 2 | No | Negative | None | DNA | 47.2 | Parapharyngeal space, petrous temporal bone | LTBR, partial mandibulectomy, FFF. Developed subsequent recurrence at petrous temporal bone and clivus. | − | + |
| 4 | 49 | M | No | Superficial parotidectomy with upper division facial nerve sacrifice | 1 | No | Negative | None | 0.8 | 14.3 | Stylomastoid foramen, EAC | Revision parotidectomy, LTBR, EAC resection, SCM flap. Developed extensive skull base recurrence with intracranial spread to MCF. | + | − |
| 5 | 79 | F | No | Total parotidectomy | 2 | Yes | Negative | Radiation | 2.6 | 4 | Stylomastoid foramen, jugular foramen | None | − | − |
| 6 | 55 | M | Yes | Total parotidectomy, upper division facial nerve sacrifice, modified radical neck dissection, mastoidectomy | 2 | Yes | Positive | Radiation | 0.9 | DNA | EAC, petrous temporal bone and mastoid, posterior cranial fossa | Subtotal TB resection, removal of sigmoid sinus and adjacent dura, trapezius flap, stereotactic radiation. | − | + |
| 7 | 61 | M | Yes | None | DNA | DNA | DNA | DNA | DNA | DNA | On original presentation - TMJ, parapharyngeal space, foramen ovale | None | + | + |
| 8 | 72 | M | No | Total parotidectomy, select neck dissection | 1 | No | Negative | None | 2.2 | 10.3 | Sphenoid sinus, cavernous sinus, clivus, parietal bone | Stereotactic radiation | − | − |
| 9 | 39 | F | No | Parotidectomy of unknown extent | DNA | DNA | DNA | None | 36.5 | 36.5 | Stylomastoid foramen | None | − | − |
| 10 | 48 | F | No | Total parotidectomy, select neck dissection, radial forearm free flap | 1 | No | Negative | None | 15.3 | 15.3 | Stylomastoid foramen | Stereotactic radiation | − | − |
DNA, data not available.
The most common presentation at the time of initial parotid tumor diagnosis was a painless mass. One patient presented with temporomandibular joint pain, trismus, and malocclusion. None of the patients presented with facial nerve weakness prior to initial parotid surgery. However, all but one of the surgically treated patients (eight of nine) acquired permanent facial weakness during the course of their treatment secondary to either tumor progression or surgical therapy necessitating facial nerve sacrifice.
All patients undergoing initial tumor resection received at minimum a superficial parotidectomy; no patients received simple enucleation. Three patients (33%) underwent additional cervical lymph node dissections or extended local resections. Two patients (22%) underwent adjuvant radiation at time of initial treatment, and no patients received adjuvant chemotherapy. Progression-free survival after initial treatment of parotid ACC for the nine surgically treated patients who developed skull base involvement ranged from 9 months to 36 years, with a median and mean of 3.2 and 8.2 years, respectively. Three patients (33%) had courses notable for three or more distinct recurrences.
Two of the 10 patients had skull base disease at initial diagnosis of primary parotid ACC, with the remainder developing cranial base involvement later in their clinical course. The time from diagnosis of parotid ACC to diagnosis of skull base invasion was variable, with a mean and median of 14.6 and 10.2 years (range 0 to 36 years), respectively. After developing skull base involvement, the seven patients who pursued further therapy underwent one or more modalities of treatment: two underwent surgery alone, one underwent surgery followed by stereotactic radiosurgery, one underwent surgery followed by both external beam radiation and stereotactic radiosurgery, two received stereotactic radiosurgery alone, and one of seven received external beam radiation alone.
The pattern of skull base invasion or metastasis was heterogeneous. The most common site for development of skull base disease was direct extension of tumor through the stylomastoid foramen. Three patients developed intracranial metastases, one with intradural and two with extradural disease. Evidence of hematogenous metastasis was seen in four of the 10 patients, all four patients developed pulmonary nodules, and two additionally developed cervical vertebral metastases. Biopsy-proven spread to the cervical lymph nodes was seen in only one patient in this series, with two other patients having radiographic evidence of cervical lymph node metastasis.
Patient 1 (below) was found to have disease that progressed from grade 2 to 3 on development of skull base disease and distant metastasis. Except for this patient, all other tumors reviewed were found to be low grade at initial excision and appeared to remain low grade despite demonstrating recurrence or invasive spread. None of the patients progressed to poorly differentiated or undifferentiated carcinoma.
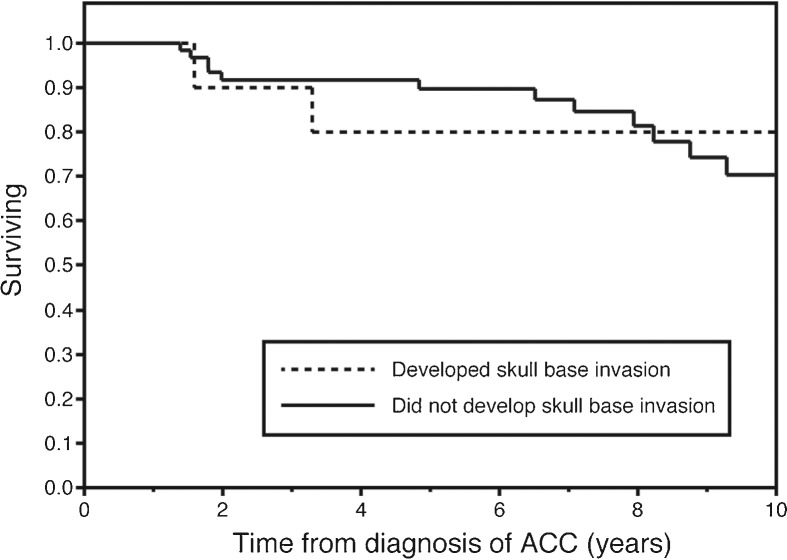
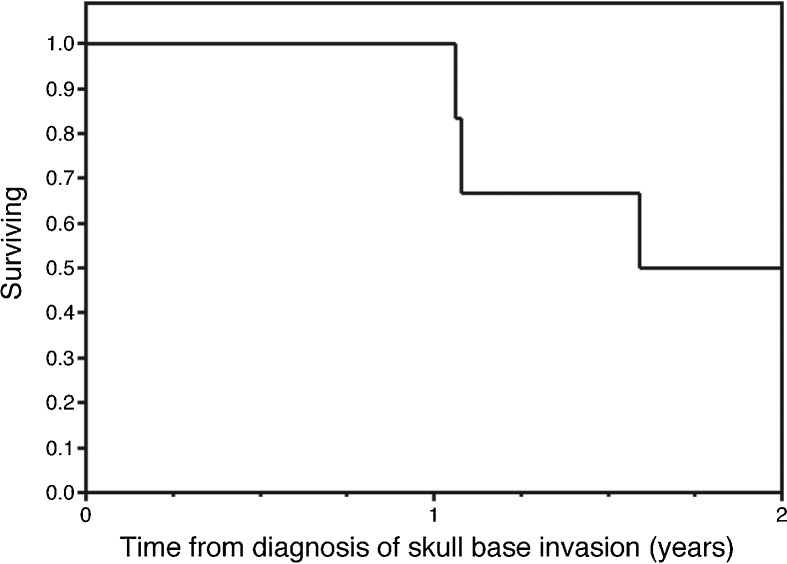
After initial diagnosis of ACC of the parotid gland, the 10-year Kaplan-Meier estimated survival for these selected patients who would go on to develop skull base involvement was 80%. This was not significantly different than 10-year survival for patients with parotid ACC without skull base involvement seen at our institution over the same period (70.4%, p = 0.86) (Fig. 1). Survival after development of skull base involvement was markedly worse, with an estimated 2-year survival of only 50% (Fig. 2). None of the subjects alive at the time of data collection were free of disease. One patient is still alive over 9 years after developing skull base disease and distant metastases, continuing to survive with slowly progressive and unresectable disease.
Figure 1.
Kaplan-Meier plot depicting overall survival after initial diagnosis of primary parotid acinic cell carcinoma. Estimated overall survival at 10 years was not significantly different between patients who developed skull base invasion (dashed line) versus those who did not (solid line) (p = 0.86).
Figure 2.
Kaplan-Meier plot depicting overall survival after development of skull base involvement by acinic cell carcinoma.
Selected Illustrative Case Narratives
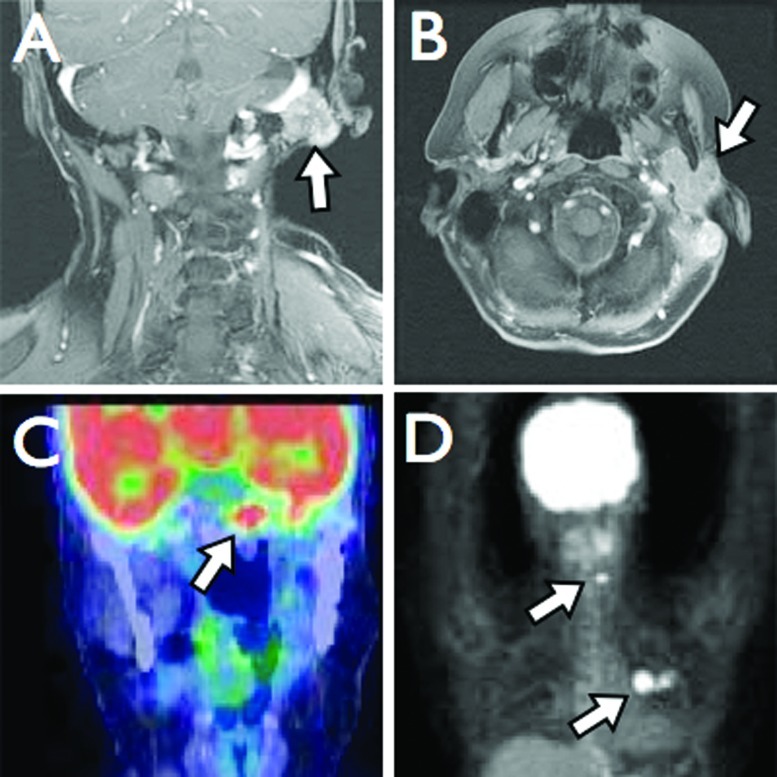
Patient 1 originally underwent a left total parotidectomy at an outside institution at 18 years of age (1992). Permanent section revealed a grade 2 ACC with a single positive deep margin. She received no adjuvant therapy. The patient developed a local recurrence 4 years later, which was treated with neutron radiation therapy, elsewhere. At age 27 (2001), she presented to our institution for the first time after she was found to have extensive local recurrence in the left masticator space, mandible, inferior alveolar nerve, and parapharyngeal space. She subsequently underwent revision parotidectomy, infratemporal fossa and parapharyngeal space dissection, and partial mandibulectomy with fibula free flap reconstruction. Margins were positive at the stylomastoid foramen and foramen ovale. She was then treated with stereotactic radiosurgery on two separate occasions for extensive skull base recurrence over the subsequent 3 years (Fig. 3A). The first treatment was in the area of the previous positive margins (left foramen ovale, Meckel's cave, and infratemporal fossa) and occurred 5 months after surgery (7.4 cm3, marginal dose 14 Gy, maximum dose 28 Gy). Two years later, she developed a left 6th nerve palsy and underwent a second treatment to the cavernous sinus (2.9 cm3, marginal dose 14 Gy, maximum dose 28 Gy). Distant metastasis to the cervical spine (C5 to C7) at age 34 (2008) was treated with surgical resection followed by stereotactic radiosurgery. She subsequently developed multiple small pulmonary metastases. Biopsy-proven metastatic disease to the contralateral sphenoid sinus and clivus was treated with stereotactic radiotherapy at age 36 (2010) (SBIMRT, 21 Gy in three fractions over 2 days). She developed a separate locoregional recurrence at the left temporomandibular joint, glenoid fossa, and external auditory canal, which was treated with external beam radiation (IMRT, 60 Gy in 50 fractions over 32 days). Later the same year she was found to have radiographic evidence of a 1.2 cm metastatic lesion along the anterior aspect of the left cerebellar hemisphere (Fig. 3B). The patient is now 37 years of age with known slowly progressive skull base disease currently being followed with serial imaging.
Figure 3.
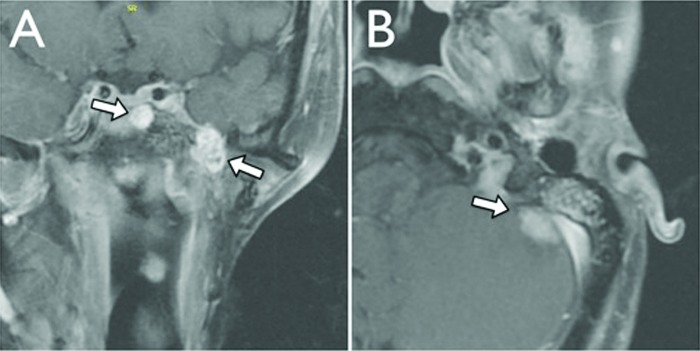
T1-weighted MRI with gadolinium demonstrating (A coronal) metastatic disease involving the left sphenoid sinus, cavernous sinus, and (B axial) left anterior cerebellar hemisphere.
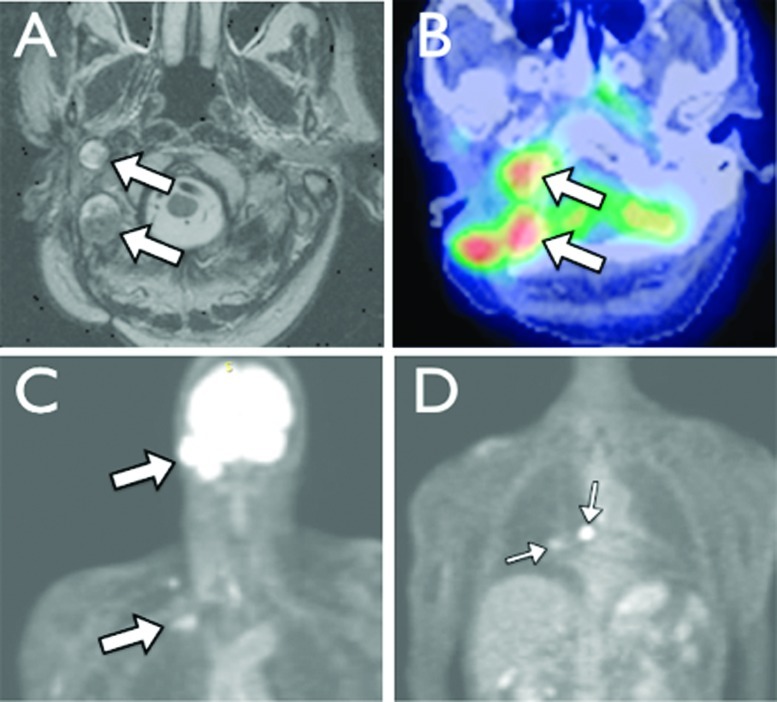
Patient 3 first developed ACC of the left parotid at the age of 18 (1961) and underwent a superficial parotidectomy at an outside institution. Over the ensuing 40 years, she underwent six additional resections as well as external beam radiation for locoregional recurrence, the first of these occurring ~7 years after initial resection. She developed biopsy-proven pulmonary metastases at age 61 (2005), for which she received chemotherapy (cyclophosphamide, doxorubicin, and cisplatin). She first presented to our institution at age 65 (2009) after developing extensive locoregional spread of disease to the temporal bone and parapharyngeal space (Fig. 4A, B). Upon evaluation she was found to have a complete left facial nerve paralysis and subsequently underwent a left subtotal temporal bone resection, partial resection of the sigmoid sinus and surrounding dura, infratemporal fossa dissection with microvascular fibular free flap reconstruction of the mandible, and dural reconstruction with a fascia lata graft. Six months later, she developed headaches, diplopia, and dysphagia. This prompted magnetic resonance imaging (MRI) and positron emission tomography-computed tomography (PET-CT), which revealed evidence of recurrence at the left temporal bone and clivus (Fig. 4C, D). Lesions consistent with distant pulmonary and cervical spine metastases were also noted. Radiation and medical oncology evaluation was recommended, but she chose not to pursue further treatment. She succumbed to disease at 66 years of age, 5 months following clinical recurrence.
Figure 4.
(A) Coronal and (B) axial T1-weighted magnetic resonance imaging (MRI) with gadolinium demonstrating involvement of the left temporal bone, sigmoid sinus, and mandible. (C) Coronal and (D) three-dimensional positron emission tomography (3D PET) imaging reveals metastases at the clivus, cervical spine, and left lung.
Patient 6 underwent near-total right parotidectomy at age 55 (2004) at an outside institution. The upper division of the facial nerve was grossly involved and was sacrificed. Final pathologic analysis revealed grade 2 ACC. He therefore underwent completion parotidectomy, right modified radical neck dissection, and mastoidectomy, and he received adjuvant external beam radiation therapy, all performed elsewhere. He developed local recurrence at the ipsilateral external auditory canal and postauricular skin and experienced progressive right-sided aural fullness 18 months following his initial operation, which prompted CT, MRI, and PET imaging (Fig. 5). This revealed recurrent disease involving the right external auditory canal, petrous and mastoid portions of the right temporal bone, posterior cranial fossa, and jugular foramen. Following radiographic evaluation, he presented to our institution for the first time and elected to undergo extensive surgical resection including a right subtotal temporal bone resection, removal of the sigmoid sinus and pre- and post-sigmoid dura, and closure of the resulting defect with a fascial allograft and trapezius myocutaneous flap. Ten months following resection (2007) he developed recurrence in the posterior fossa bed and new lung lesions suspicious for distant metastatic disease on PET imaging. He underwent stereotactic radiosurgery (30.9 cm3, marginal dose 15 Gy, maximum dose 30 Gy) to the skull base, which failed to obtain local control. He was referred to medical oncology but deferred further treatment and expired only 7 months later.
Figure 5.
(A) Axial T1-weighted magnetic resonance imaging (MRI) with gadolinium and (B) positron emission tomography-computed tomography (PET-CT) fusion study demonstrating multinodular disease at the right base of skull. Coronal PET imaging revealing (C) locoregional recurrence at the right base of skull with right supraclavicular and (D) pulmonary metastasis.
Discussion
Once considered benign, ACC is now widely recognized as a malignant disease entity with the capability for local invasion and distant metastasis. The basic principles of obtaining clear surgical margins, providing radiotherapy for residual or unresectable disease, and preservation of vital structures whenever possible are all applicable to salivary gland malignancies, including ACC.9 However, the anatomical challenges of accessing the skull base combined with the slow-growing nature of ACC can make diagnosis and treatment of recurrent disease particularly difficult, leading to multiple recurrences that cannot be managed definitively by resection, radiation, or chemotherapy. This current series of patients highlights several of these management challenges and current controversies regarding treatment of ACC.
Several previous studies have confirmed the unique natural history of ACC.1,2,6,9,10,12,13,14 Overall, it is the most survivable of the salivary gland malignancies, with a 5-year disease-specific survival rate of 91%.9 Most cases carry a favorable prognosis, without need for elective neck dissection or adjuvant therapy, but rare cases of invasive, aggressive, and multiply recurrent disease are seen. These recurrences can sometimes be seen decades after initial tumor resection, a finding that has been confirmed by previous studies. Lewis et al demonstrated that even amongst patients who undergo “modern treatment” (i.e., some form of parotidectomy), the determinate survival curve does not level off until 17 years after initial therapy. These authors reported an estimated overall death from disease rate of ~13%, but older studies had reported rates as high as 26%. The excellent survival rate, even for those who develop recurrent disease, might bias a clinician toward a less aggressive follow-up schedule. However, the propensity for delayed and potentially fatal recurrence makes long-term (i.e., >10-year) follow up seem critical. Our findings from this current series seem to confirm this, as the median time from initial diagnosis of ACC to development of skull base disease in the currently presented patients was greater than 10 years. Once skull base invasion occurred, however, overall survival declined dramatically.
The true incidence of skull base involvement after development of parotid ACC is difficult to determine. Although 10 of the 87 patients (11.5%) evaluated at our institution for parotid ACC over the past 16 years developed skull base involvement, the tertiary referral pattern of our practice certainly inflates this number. Of these 10 cases, seven were seen at our institution after developing recurrent disease following treatment of their primary parotid tumor elsewhere.
To help identify patients at greater risk for disease progression who will need more extensive follow up, several studies have focused on identifying factors that may predict poor outcomes. Perzin et al reviewed 51 cases of ACC and found that a painful mass on presentation, tumor fixation on clinical examination, facial nerve involvement, deep lobe involvement, positive surgical margins, cellular atypia, and size of original tumor may confer a poorer clinical course.13 In a more modern series by Gomez and colleagues, a series of 35 patients with parotid ACC was reviewed. Extracapsular extension (gross or microscopic), facial nerve sacrifice at time of surgery, lymph node involvement, positive surgical margins, and high pathologic grade as evidenced by >2 mitoses/10 high-power fields, necrosis, or pleomorphism were all associated with decreased disease-free and overall survival. Perineural and vascular invasion were associated with decreased disease-free survival only.10 A previous review of the Mayo Clinic experience with ACC identified evidence of gross invasion at time of surgery as well as microscopic evidence of atypia, increased mitotic figures, or desmoplastic stromal reaction as predictive of future disease progression.9 Taken as a whole, these reports suggest that grade appears to play an important role in predicting disease-free and overall survival. This finding was not necessarily reproduced in our study, however, as patients with low-grade tumors were seen to have progressive and fatal skull base disease.
A universally agreed upon grading system for ACC has yet to emerge, making it difficult to assign more precise prognostic value to histopathologic findings.10 Given this lack of consensus and the retrospective nature of this review, there may have been some small disagreements in exact choice of grade between the pathologists who reviewed these tumors over the years. However, the pathologic distinction between a low-grade (1 or 2) versus a poorly differentiated high-grade tumor should not be subtle. All of the tumors in this series were found to be low grade at the time of initial excision, with only one case demonstrating tumor grade progression on review of subsequent pathologic specimens from later tumor excisions. Clearly, this demonstrates that high tumor grade is not necessary for development of local invasion or distant metastasis. This also confuses the picture as to which patients should receive adjuvant radiation therapy, as only high-grade tumors have been definitely identified as appropriate candidates for adjuvant radiation therapy in previous studies.15
Facial nerve involvement, as evidenced by facial weakness or tumor invasion found at the time of surgery, is a common finding in patients with ACC and has been variably associated with disease progression. In the currently presented case series, no patients had facial nerve symptoms prior to their initial surgery. However, three (of nine) patients had tumor immediately adjacent to the facial nerve or underwent partial facial nerve sacrifice due to tumor invasion at the time of initial surgery. An additional two patients underwent partial facial nerve sacrifice during subsequent operations. Two patients developed disease involving the trigeminal nerve at the foramen ovale. Given this high frequency of nerve involvement, it is not surprising that direct extension of tumor to the region of the stylomastoid foramen was the most common pattern of skull base disease in these patients. Propensity for other parotid malignancies, such as adenoid cystic carcinoma, to spread along neural sheaths through bony foramina has been previously demonstrated.3 Although perhaps less common, this case series suggests that ACC is capable of similar patterns of neurotrophic spread.
At present, there is no strong consensus regarding required extent of primary surgery for ACC confined to the parotid gland. Larger retrospective series have demonstrated a slightly lower rate of tumor recurrence in patients who undergo total parotidectomy compared with those who undergo only superficial parotidectomy. Rates of metastasis and death from disease were similar in these two patient groups, though selection bias for patients with larger tumors undergoing total parotidectomy may have influenced these results.9 Some authors have recommended that patients with parotid ACC undergo total parotidectomy with attempt to preserve the facial nerve,5,16 whereas others argue that superficial parotidectomy alone with facial nerve preservation is sufficient for well-circumscribed tumors located in the superficial lobe.2,6,9 Tumor enucleation should be avoided, as significantly higher recurrence rates have been demonstrated with this.9 If the mass grossly involves surrounding extraparotid structures, marginal biopsies may be obtained to help guide extended dissection. Based on personal or institutional experiences, many investigators have concluded that sacrifice of a functional facial nerve is only necessary when gross invasion of perineural structures is present.
A clear set of indications for adjuvant radiation for primary parotid ACC has not yet emerged. In this series, two (of nine) patients underwent adjuvant radiation after initial excision, both for the indication of extracapsular spread of tumor, and one additionally had suspected residual disease at the stylomastoid foramen. Patient 1 had positive margins at the time of initial excision but received no adjuvant treatment immediately after resection.
More time will be needed to better ascertain the survivability of skull base involvement of ACC. The estimated overall survival at 2 years after development of skull base disease was 50%, despite the majority of the patients undergoing aggressive multimodality treatment. Two patients were lost to follow up immediately after diagnosis and two others have less than 1 year of follow-up time. One patient is still alive more than 9 years after development of skull base disease, suggesting that long-term survival, while rare, is possible. Younger and otherwise healthy patients with single-focus recurrences without involvement of critical vascular structures should be considered surgical candidates, but given the generally poor outcomes with skull base ACC, enthusiasm for aggressive surgical intervention should be tempered. Stereotactic radiosurgery should be considered for patients with less-favorable tumors.
Conclusion
ACC is a relatively uncommon salivary gland malignancy that often follows a less-aggressive clinical course. A small group of patients will develop skull base involvement, often in a delayed fashion (>10 years), where cure is unlikely and disease control is difficult. Patients who develop cranial base disease are at a high risk for multiple late recurrences and death from disease. These observations underscore the importance of long-term follow up in this unique patient group.
Footnotes
Note Financial & material support: No funding or other support was required for this study. Author(s) conflict of interest to declare: none This manuscript has not been previously published in whole or in part or submitted elsewhere for review. IRB Approval: 09–003999
References
- 1.Hoffman H T, Karnell L H, Robinson R A, Pinkston J A, Menck H R. National Cancer Data Base report on cancer of the head and neck: acinic cell carcinoma. Head Neck. 1999;21:297–309. doi: 10.1002/(sici)1097-0347(199907)21:4<297::aid-hed2>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 2.Spafford P D, Mintz D R, Hay J. Acinic cell carcinoma of the parotid gland: review and management. J Otolaryngol. 1991;20:262–266. [PubMed] [Google Scholar]
- 3.Leonetti J P, Marzo S J, Agarwal N. Adenoid cystic carcinoma of the parotid gland with temporal bone invasion. Otol Neurotol. 2008;29:545–548. doi: 10.1097/MAO.0b013e318168da96. [DOI] [PubMed] [Google Scholar]
- 4.Martinez-Devesa P, Barnes M L, Milford C A. Malignant tumors of the ear and temporal bone: a study of 27 patients and review of their management. Skull Base. 2008;18:1–8. doi: 10.1055/s-2007-992766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vidyadhara S, Shetty A P, Rajasekaran S. Widespread metastases from acinic cell carcinoma of parotid gland. Singapore Med J. 2007;48:e13–e15. [PubMed] [Google Scholar]
- 6.Kim S A, Mathog R H. Acinic cell carcinoma of the parotid gland: a 15-year review limited to a single surgeon at a single institution. Ear Nose Throat J. 2005;84:597–602. [PubMed] [Google Scholar]
- 7.Prasad M, Kraus D H. Acinic cell carcinoma of the parotid gland presenting as an external auditory canal mass. Head Neck. 2004;26:85–88. doi: 10.1002/hed.10347. [DOI] [PubMed] [Google Scholar]
- 8.Kinney S E, Wood B G. Malignancies of the external ear canal and temporal bone: surgical techniques and results. Laryngoscope. 1987;97:158–164. doi: 10.1288/00005537-198702000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Lewis J E, Olsen K D, Weiland L H. Acinic cell carcinoma. Clinicopathologic review. Cancer. 1991;67:172–179. doi: 10.1002/1097-0142(19910101)67:1<172::aid-cncr2820670129>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 10.Gomez D R, Katabi N, Zhung J. et al. Clinical and pathologic prognostic features in acinic cell carcinoma of the parotid gland. Cancer. 2009;115:2128–2137. doi: 10.1002/cncr.24259. [DOI] [PubMed] [Google Scholar]
- 11.Douglas J G, Goodkin R, Laramore G E. Gamma knife stereotactic radiosurgery for salivary gland neoplasms with base of skull invasion following neutron radiotherapy. Head Neck. 2008;30:492–496. doi: 10.1002/hed.20729. [DOI] [PubMed] [Google Scholar]
- 12.Ellis G L, Corio R L. Acinic cell adenocarcinoma. A clinicopathologic analysis of 294 cases. Cancer. 1983;52:542–549. doi: 10.1002/1097-0142(19830801)52:3<542::aid-cncr2820520326>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 13.Perzin K H, LiVolsi V A. Acinic cell carcinomas arising in salivary glands: a clinicopathologic study. Cancer. 1979;44:1434–1457. doi: 10.1002/1097-0142(197910)44:4<1434::aid-cncr2820440439>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 14.Levin J M, Robinson D W, Lin F. Acinic cell carcinoma: collective review, including bilateral cases. Arch Surg. 1975;110:64–68. doi: 10.1001/archsurg.1975.01360070064011. [DOI] [PubMed] [Google Scholar]
- 15.Batsakis J G, Luna M A, el-Naggar A K. Histopathologic grading of salivary gland neoplasms: II. Acinic cell carcinomas. Ann Otol Rhinol Laryngol. 1990;99:929–933. doi: 10.1177/000348949009901115. [DOI] [PubMed] [Google Scholar]
- 16.Federspil P A, Constantinidis J, Karapantzos I, Pahl S, Markmann H U, Iro H. [Acinic cell carcinomas of the parotid gland. A retrospective analysis] HNO. 2001;49:825–830. doi: 10.1007/s001060170031. [DOI] [PubMed] [Google Scholar]