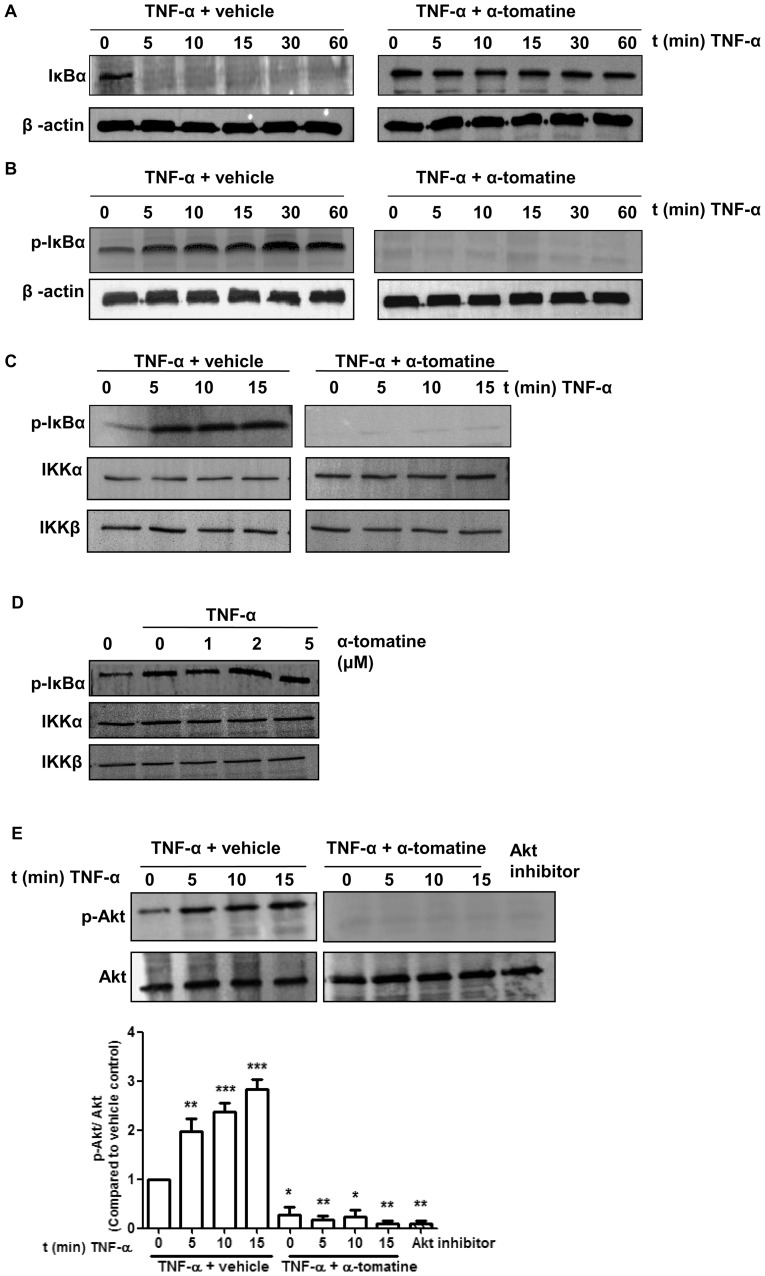
Figure 2. Effect of α-tomatine on IκBα Kinase activity.
(A) Cells were grown to 70–80 % confluence, treated with either 0.1% DMSO (vehicle) or 2 µM α-tomatine in DMSO for 30 minutes, followed by treatment with 10 ng/ml of TNF-α for the indicated times. The presence of IκBα was detected by Western blot analysis. (B) To determine whether α-tomatine inhibits IκBα degradation by blocking IκBα phosphorylation, cells were treated with either 0.1% DMSO (vehicle) or 2 µM α-tomatine in DMSO for 30 minutes, followed by 50 µg/ml calpain inhibitor ALLN for 30 minutes, and then treated with 10 ng/ml of TNF-α for the times indicated. Anti-phospho-IκBα Western blot analysis was performed on cytoplasmic extracts. β-actin served as a loading control. (C) PC-3 cells were preincubated with either 2 µM α-tomatine or 0.1% DMSO (vehicle) for 30 minutes, and then treated with 10 ng/ml TNF-α for the indicated times. IKKα and IKKβ were immunoprecipitated from lysates from cells and in vitro kinase assays were performed using GST-IκBα as substrate as described in “Materials and Methods”. Western blot analysis was performed to detect phosphorylated IκBα. (D) IKK complex immunoprecipitated from vehicle and TNF-α-treated PC-3 cell extracts with an anti-IKKα and IKKβ antibodies was assayed for IKK activity. The kinase reaction mixture was incubated with α-tomatine as indicated. The expressions of phosphorylated IκBα and IKK were examined by Western blot analysis using anti-phospho-IκBα, anti-IKKα and anti-IKKβ antibodies. To examine the basal level of expression of IKK proteins, whole-cell extracts analyzed by Western blotting using anti-IKKα and anti-IKKβ antibodies. (E) PC-3 cells were pretreated with 2 µM α-tomatine for 30 minutes, and then treated with 10 ng/ml TNF-α for the indicated times. Lysates extracted from cells treated with 10 µM Akt inhibitor VIII for 3 hours serve as inhibition control. Cytoplasmic extracts were used for Western blotting using anti-phosphospecific Akt (Ser473) antibody. The same blot was reprobed with nonphosphorylated Akt antibody. Graphical representation of densitometry analysis of phosphor-Akt Western blot analysis from three independent experiments is shown below the panel. The ratio of the signal intensity of each protein to loading control was normalized to the vehicle control. * P<0.05, ** P<0.01, *** P<0.001 vs vehicle control.