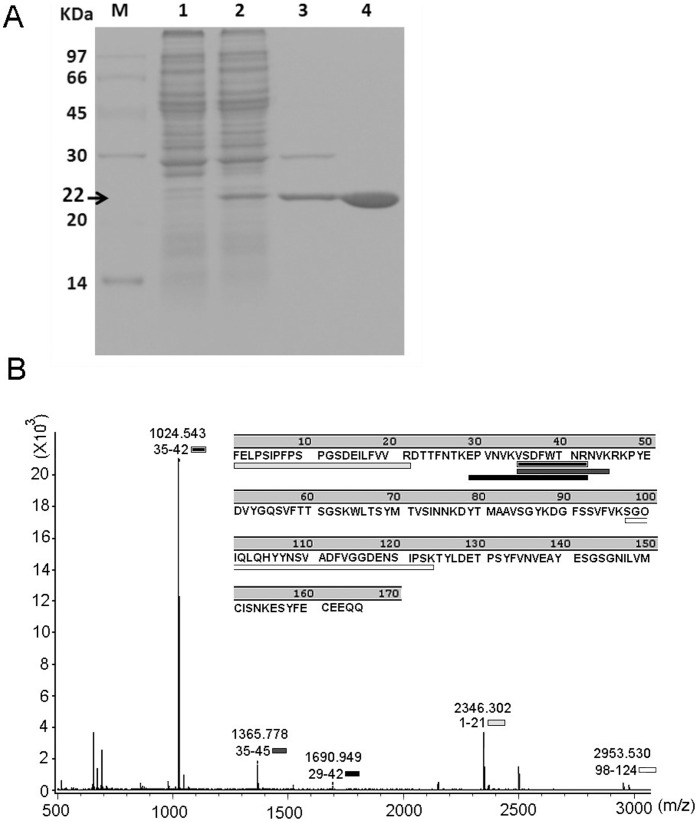
Figure 1. Identification of Gh-rTDH purified from G. hollisae.
(A) SDS-PAGE analysis of Gh-rTDH. Marker proteins (M): phosphorylase b (97 kDa), albumin (66 kDa), ovalbumin (45 kDa), carbonic anhydrase (30 kDa), trypsin inhibitor (20 kDa), and α-lactoalbumin (14 kDa); lane 1: cell crude extract of BL21(DE3) pLysS containing the pCR2.1-TOPO plasmid alone; lane 2: crude protein expression in BL21(DE3) pLysS containing pCR2.1-TOPO-Gh-tdh; lanes 3 and 4: Phenyl Sepharose 6 Fast Flow purification yielded a homogenous protein with a molecular mass of ∼22 kDa. (B) The tandem mass spectrum of the doubly charged tryptic peptide at m/z 1024.543 from the SDS-PAGE of Gh-rTDH revealed a unique hit matching 35VSDFWTNR42 of the Gh-rTDH peptide sequence.